Introduction
Drug-induced liver injury (DILI) is a critical condition among adverse drug reactions (ADRs), which accounts for approximately 10% of all cases of acute hepatitis and is the most common cause of acute liver failure in the United States1,2). The incidence of DILI has been estimated as an annually 14 ± 2.4 per 100,000 general populations worldwide3), and was approximately 0.7% to 1.7% among inpatients4). A broad range of drugs are known to can induce liver damage, including anesthetics, anticancer drugs, antibiotics, antiretrovirals, and cardiac medications5,6).
On the other, tuberculosis (TB) remains one of the most important public health challenges in the world, and Korea still has an intermediate tuberculosis burden7). The standard anti-TB therapy usually shows the good response, hepatotoxic side effects are serious challenges in patients receiving anti-TB treatment8). DILI is one of the most deleterious side effects associated with chemotherapy against tuberculosis. One study reported 12.9% of DILI rate in patients received standard anti-TB therapy in China9).
Recently herbal drugs are often considered as a major cause of hepatotoxicity10), which would distort reputation for herbal medicine in Korea. The major studies on DILI even for herbal medicine-associated toxicity were conducted in western medicine field to date11,12). The more active roles in issues of herbal drug safety as well as in management of DILI are required to physicians or researcher of Traditional Korean medicine (TKM).
This study aims to report a woman case with severe DILI by anti-TB agents, which was recovered by TKM-based treatments.
Report of the case
1. Medical history and examination
A 45-year-old woman has been healthy except a rare lower abdomen pain and diarrhea after certain foods. She hasn’t used alcohol, and has no past or family history of hepatic disease or tuberculosis. She had 25.3 body mass index (BMI) with slight abdomen obesity. She was an office worker along doing homework. During medical check for her abdominal pain, she was diagnosed with intestinal tuberculosis, and got a prescription of anti-TB from January 2016 (Table 1). On approximately 2 months of drug administration, she had felt sever abdominal discomfort, dyspepsia, indigestion and fatigue after moderate psychiatric stress episode for 2 days. Acupuncture treatment in a local oriental clinic slightly reduced her symptoms temporarily, but she visited an Oriental hospital because of same level of complaints on next day. Based on her medical history of taking anti-TB drug, a laboratory test was conducted. According to the notably elevated levels of serum alanine aminotransferase (ALT, 1,212 IU/L) and aspartate aminotransferase (AST, 584 IU/L), gamma glutamyl transpeptidase (GGT, 161 IU/L), alkaline phosphatase (ALP, 100 IU/L), and total bilirubin (0.9 mg/ml), she was hospitalized.
2. Treatments and clinical outcome
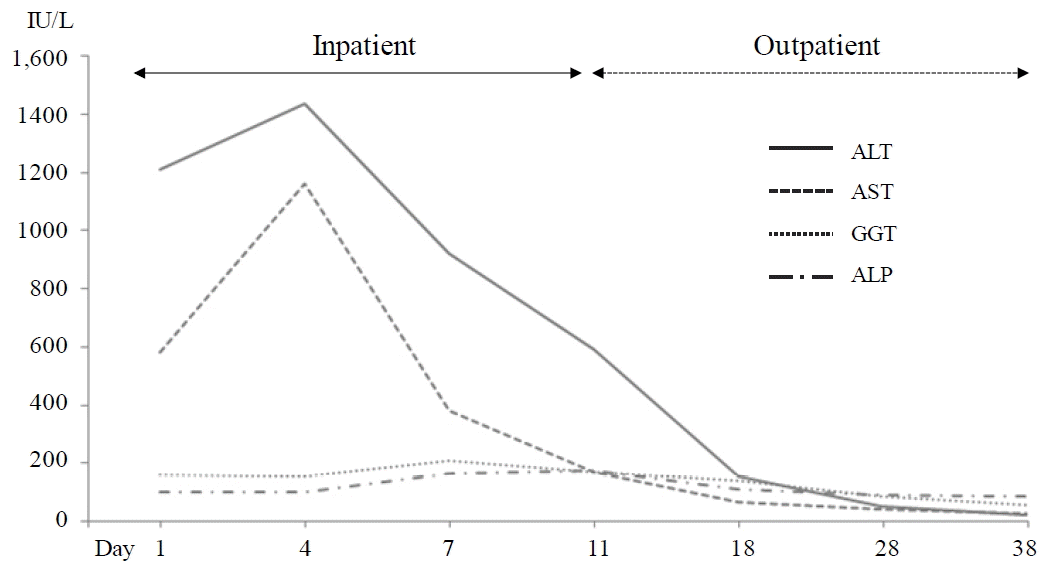
Along with stopping the anti-TB drugs, she was given with indirect moxibustion (CV8), acupuncture (mainly at CV12 and bilateral ST36, LI4 and LR3) and an herbal drug (Chungganplus syrup, two packs per day, Table 2) as an inpatient. The subjective symptoms including abdominal discomfort, dyspepsia, indigestion and fatigue gradually become improved from 3rd day of hospitalization, but continued as mild by 10th day of hospitalization. The serum levels of AST and ALT further elevated approximately over 25 times of normal upper limit on 3rd day of hospitalization (ALT 1,435 IU/L, AST 1,159 IU/L, ALP 100 IU/L and GGT 157 IU/L respectively). The hepatic enzymes were gradually lowered by ALT 592 IU/L, AST 169 IU/L, ALP 175 IU/L and GGT 171 IU/L on 11th day of hospitalization, when she left the hospital. On 39th day of initial treatment, all hepatic enzymes became normal range (Fig. 1). The serum total bilirubin level was within normal range throughout treatment. The tests for viral infections (HBs Ab, HCV Ab and HVA Ig M) were negative (data not shown), and abdominal ultrasonography revealed normal features (Fig. 2).
Discussion and Conclusion
In this case, the patient had intestinal tuberculosis, an extrapulmonary tuberculosis (EPTB) that constitutes about 20% of all cases of TB in Korea16). The Joint Committee for the Development of Korean Guidelines for Tuberculosis published Korean Guidelines for Tuberculosis in 2011, and then this patient administrated the first-line oral anti-TB drugs (Isoniazid, rifampin, ethambutol)17). This patient showed a typical feature of acute liver injury; troubles in gastric function with a severe fatigue, but without jaundice. According to Roussel Uclaf Causality Assessment Method (RUCAM)18), the RUCAM score of this case was 11. The medical examinations in laboratory tests and radiography excluded other liver disorders likely viral hepatitis.
Contrary to general ADR presenting a dose dependent and predictable characteristic, DILI shows usually idiosyncratic metabolic responses that are the dose independent and unpredictable patterns19). The most current guidelines recommend the same regimen for both EPTB and pulmonary TB, and this regimen induces a wide incidence range of hepatotoxicity from 8% to 18%20–22). Particularly isoniazid brings hepatotoxicity risk from 10% to 20% for asymptomatic elevation of serum liver enzyme, and severe and sometimes fatal hepatitis as 0.3% in people 21 to 35 years old and over 2% in those over age 5023). The age, hypoalbuminemia, and regular alcohol intake are risk factors for the development of DILI by anti-TB drugs24), which none of them belonged to the patient of this case.
DILI pathological feature is generally classified into three types; hepatocellular, cholestatic, and mixed type, which is defined as a rise in either ALT or ALP level25). The hepatocellular type is more common in younger patients, whereas cholestatic pattern of DILI increases with older age 26). For this case, the fold ratio of the elevated ALT value by upper limit of normal (ULN) to the elevated ALP value by ULN was 30 (R > 2) which indicated a hepatocullular pattern of hepatotoxicity. The typical biomarkers for cholestatis including bilirubin, ALP and GGT were normal range. Current case showed a severe liver injury (ALT >1000 IU/L). The incidence rate of this severe DILI by anti-TB drugs was between 0.1 to 0.5% in a Japanese study27).
A Korean study presented that aapproximately 70% of the cases of DILI occurred in the first month of antituberculous treatment28). This patient therefore needed the monitoring of hepatotoxicity at first month after initiation of antituberculous treatment; however it was not for her. The principle of management for DILI is to stop all anti-TB medications when elevation in ALT reaches 3 times ULN with symptoms or > 5 times ULN, and then non-hepatotoxic anti-TB drugs should be chosen29). In general, 5% to 15% of DILI patients had chronic DILI and persistent laboratory abnormalities30,31). One Korean study revealed 1.8% of deaths or transplantations among 371 hospitalized cases with DILI32). The patient of this case had recovered the both subjective symptoms quickly and biochemical abnormality within 30 days.
In current case, patient was cared with TKM-based therapies including herbal medicine (Chungganplus syrup) without any conventional dedication.
Recently the herbal medicine is concerned often as a leading cause of DILI in Korea3). There are however concerning the exaggeration for the possibility of herbal medicine-associated hepatotoxicity, leading to distortion of reputation for herbal medicine in Korea33). Traditional Korean medicine doctors need to play a leading role in drug safety study especially for herbal medicine; however, researchers in western medicine field have conducted the major studies even for herb-related DILI so far.
This report presented a severe case of DILI by conventional drugs, recovered in Oriental hospital. This study would be helpful to provide the practical knowledge of anti-TB drug-induced hepatotoxicity and information to care the patient with DILI in TKM field.