Observation of Inflammatory Marker Levels in Sprague-Dawley Rats with Youngyopaedoc-san-related Anti-atherogenic Effect
Article information
Abstract
Objectives:
Sprague-Dawley rats were fed with high fat diet, and atherogenic changes were seen in the aorta. However, when Sprague-Dawley rats were fed with a high fat diet and administered Youngyopaedoc-san together, these atherogenic changes were rarely seen. This study was aimed to find the inflammatory marker level changes in Sprague-Dawley rats with Youngyopaedoc-san-related anti-atherogenic effect.
Methods:
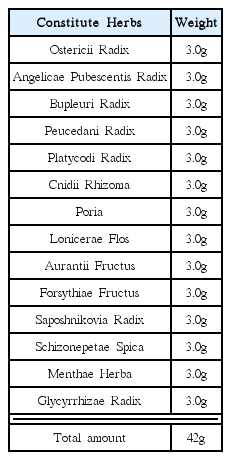
The extract from Youngyopaedoc-san was made by the pharmacy department of Kyung-hee Oriental Medical Hospital. The animals were divided into five groups: normal diet, high fat diet, high fat diet with Youngyopaedoc-san, high fat diet with Vytorin, and high fat diet with Youngyopaedoc-san and Vytorin. A light microscopic image of a cross section taken from the aorta of the Sprague-Dawley rat was analyzed. We compared inflammatory marker levels among the five groups.
Results:
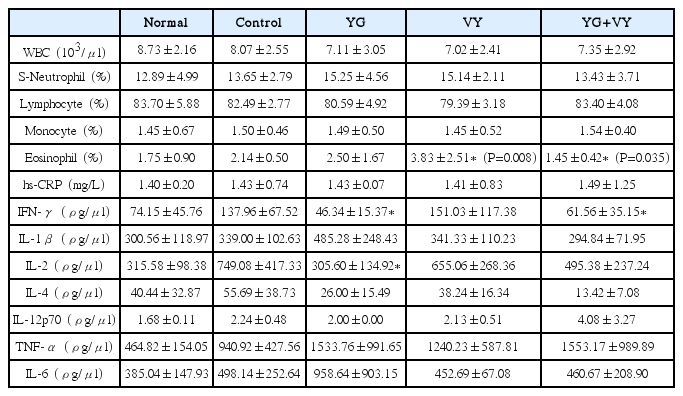
The complex of Youngyopaedoc-san and Vytorin has more anti-atherogenic effects in the aorta of Sprague-Dawley rats fed with high fat diet than Vytorin alone. Youngyopaedoc-san has inhibitory effect on the increase of IFN-ɣ and IL-2 levels. The difference on eosinophil levels of each group was statistically significant, but the eosinophil level of each group was within normal limits, so the difference on eosinophil levels was not clinically significant.
Conclusions:
Youngyopaedoc-san-related anti-atherogenic effect could be a result of inhibitory mechanism on IFN-ɣ and IL-2.
The light microscopic images of a cross section taken through the wall of the aorta of Sprague Dawley rats.
As shown in (A) and (B), the innermost layer, the endothelial cells are supported by a thin bed of subendothelial connective tissue that rest on thick sheet of elastic fiber. In (C) and (D), The arrow shows subendothelial foam cell deposits. In (E), The arrow shows the tunica intima composed of endothelial cells and elastic lamina tightly. The foamy vacuolization and fat deposits in the tunica media mean degenerative changes of wall. In (F), The arrow shows degenerative chages in the tunica media at the bifurcation of aorta. In (G), The arrow shows the foamy vacuolization in the tunica intima apparently. In (H), The arrow shows the vacuolization and the fat infiltration in the tunica media. In (I), The arrow shows minor fat deposits in the subendothelial layer. In (J), The arrow slightly shows minor vacuolizaion in the endothelial layer. It is almost normal. Animals were divided into five groups: Normal, fed with normal diet; Control, fed with high fat diet; YG, fed with high fat diet with Youngyopaedoc-san; VY, fed with high fat diet with Vytorin; and YG+VY, fed with high fat diet with Youngyopaedoc-san and Vytorin.