Effects of topical application of realgar on pruritus and inflammation of atopic dermatitis
Article information
Abstract
Objectives:
Realgar has been frequently used for skin disorders in history of herbal medicine. However, the efficacy of realgar has not been examined in atopic dermatitis (AD). In this study, the effects of realgar on AD were investigated, especially on pruritus and inflammation.
Methods:
AD lesions were induced in the shaved backs of BALB/c mice through repeated application of DNCB. The mice were treated for 11 days with 1% realgar (100 μL/day). Histological changes in skin thickness were observed. The anti-pruritic effects of realgar were evaluated by the change in numbers of scratching behavior of mice and expression of substance P. The expressions of cytokines IL-4 and IL-6 were measured. Also, anti-inflammatory effects of realgar were examined on expressions of NF-κB, phospho-I κB α and mitogen-activated protein kinases (MAPKs).
Results:
Realgar decreased skin thickness (both dermal and epidermal) 38% and 17% respectively, compared to positive control, DNCB group. The scratching behavior of mice was reduced by 42% and expression of substance P was significantly less. Cytokines IL-4 and IL-6 were significantly reduced by 52.6% and 77.6%, respectively. The expressions of NF-κB, phospho-I κBα and MAPKs (phospho-ERK1/2, -p38 and -JNK) were significantly suppressed with marked effects on phospho-ERK1/2.
Conclusions:
The collective results suggest that realgar shows anti-pruritic and anti-inflammatory effects on AD. And realgar might be a potential therapeutic candidate for treatment of atopic dermatitis.
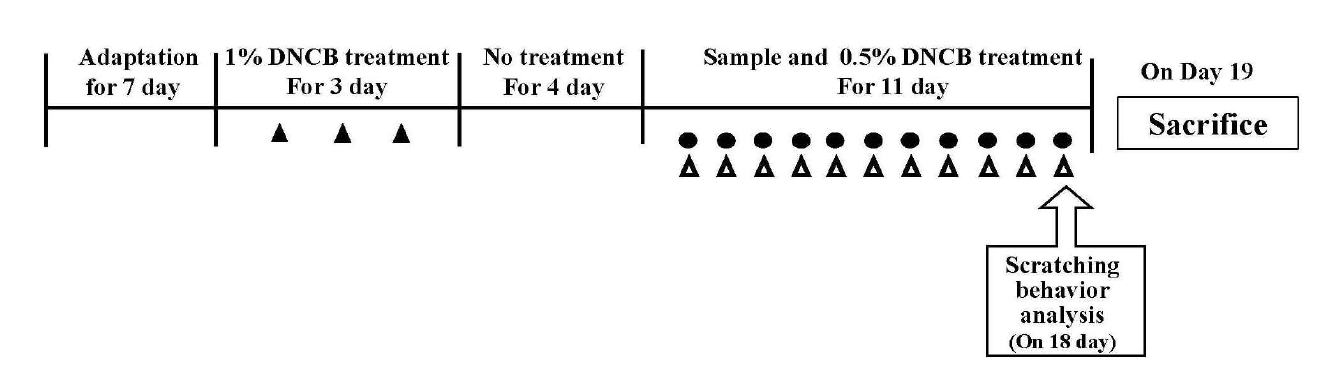
Experimental schedule. ▲: The shaved dorsal skin was applied with 100 µL of 1% DNCB solution (in acetone: olive oil=4:1) for sensitization, ●: The realgar group were treated 1% realgar (5 mg/day) and the DEX group treated 100 µL dexamethasone (10 uM, dissolved in PBS), △: 100 µL of 0.5 % DNCB solution were applied on the dorsal skin.
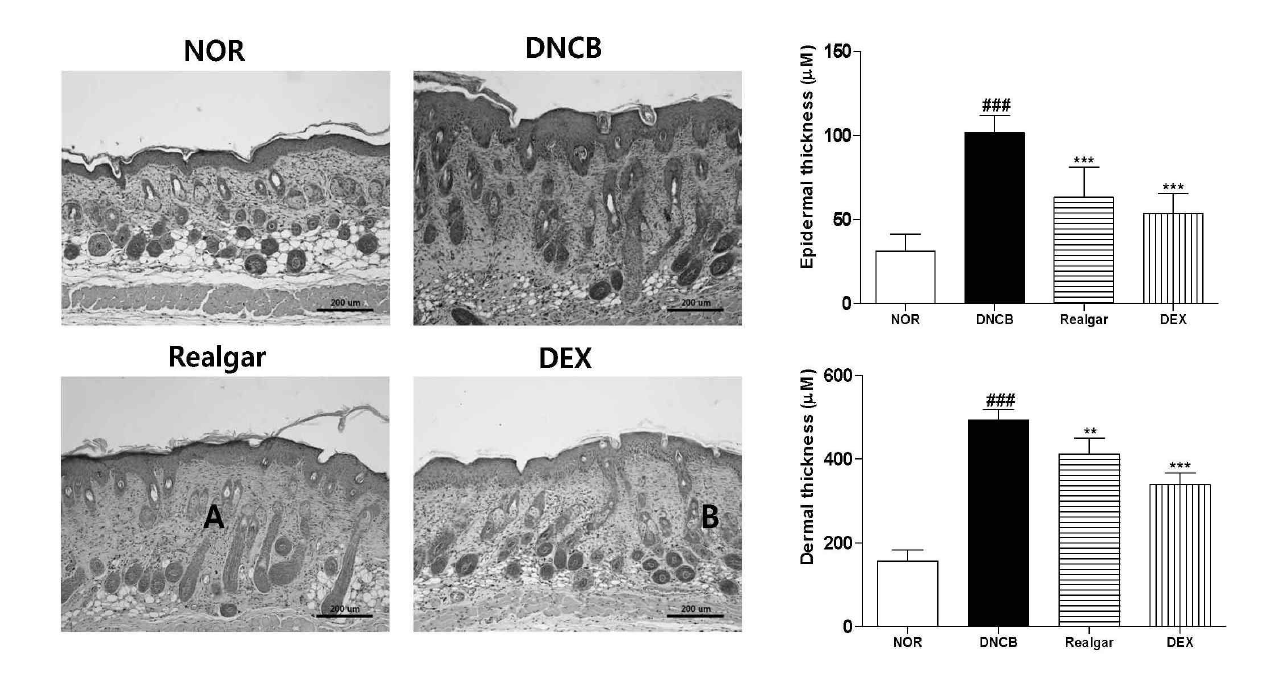
Change in epidermis and dermis upon application of realgar. (A) The thickness of epidermis, (B) The thickness of dermis. Hematoxylin-eosin staining showed a decrease thickness of epidermis and dermis in the realgar-treated group and DEX group. Results shown are representative of five observations. Data are presented as means ± SD and analyzed with ANOVA. ###P < 0.001, versus the NOR group. ***P < 0.001, versus the DNCB group. Magnification : X 100.

Changes in scratching behavior upon application of realgar. Scratching behavior of mouse was checked after 1 h had passed following the last application of DNCB (on 18 day). Each mouse of all group were videotaped for 20 min to count the number of scratching behavior. Data are presented as means ± SD (n = 5) and analyzed with ANOVA. ###P < 0.001, versus the NOR group. **P < 0.05, versus the DNCB group.
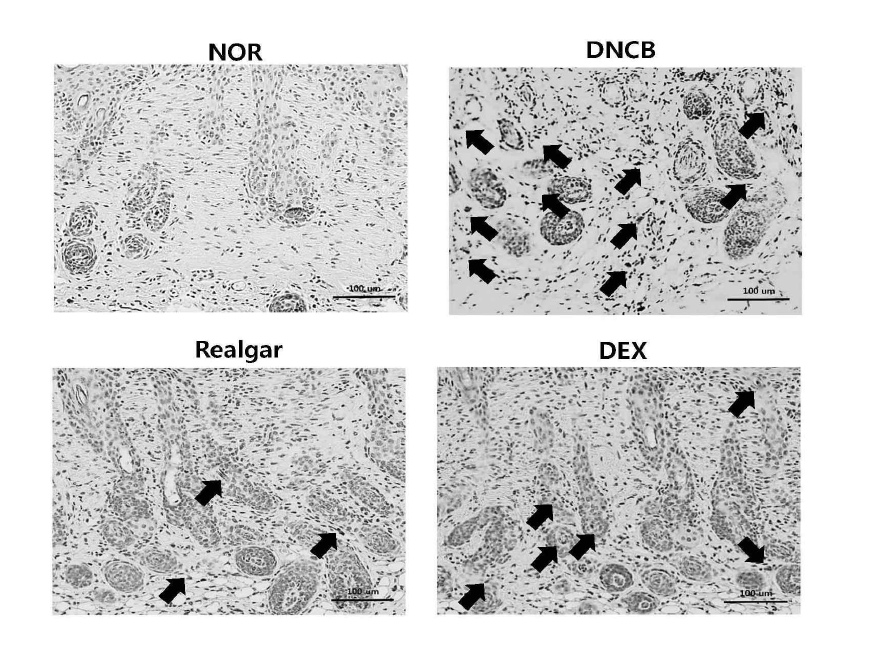
Changes in substance P expression upon application of realgar. The expression of substance P was confirmed by Immunohistochemistry staining. Skin specimens were excised 12 h after the final DNCB challenge. The arrow indicates the expression of substance P. Magnification : X 200.
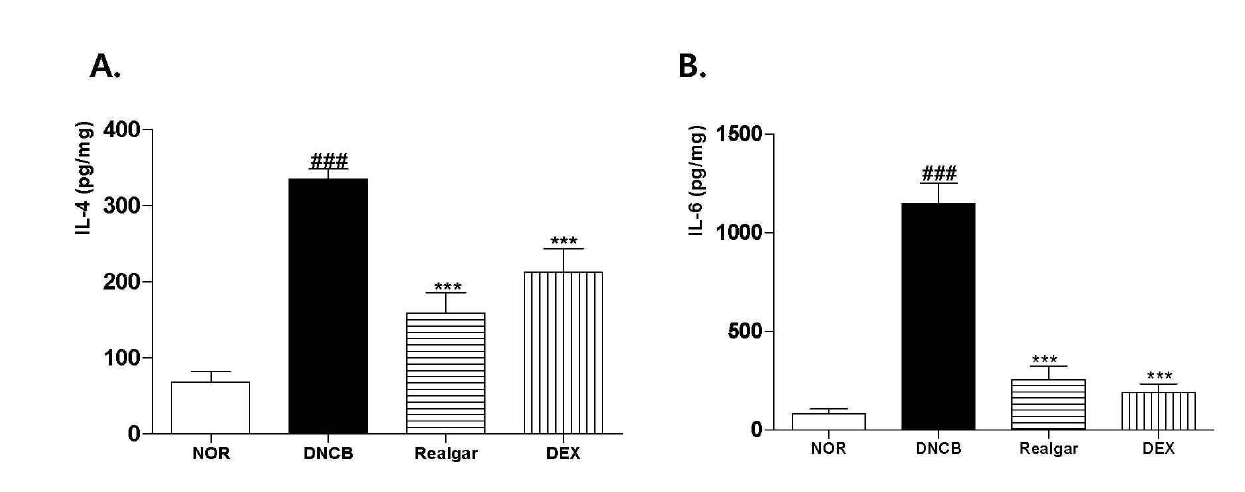
Changes in cytokine levels upon application of realgar. The cytokine levels of IL-4 and IL-6 were measured in the dorsal skin using mouse ELISA kits. Data are presented as means ± SD (n = 5) and analyzed with ANOVA.###P < 0.001, versus the NOR group. ***P < 0.001, versus the DNCB group.
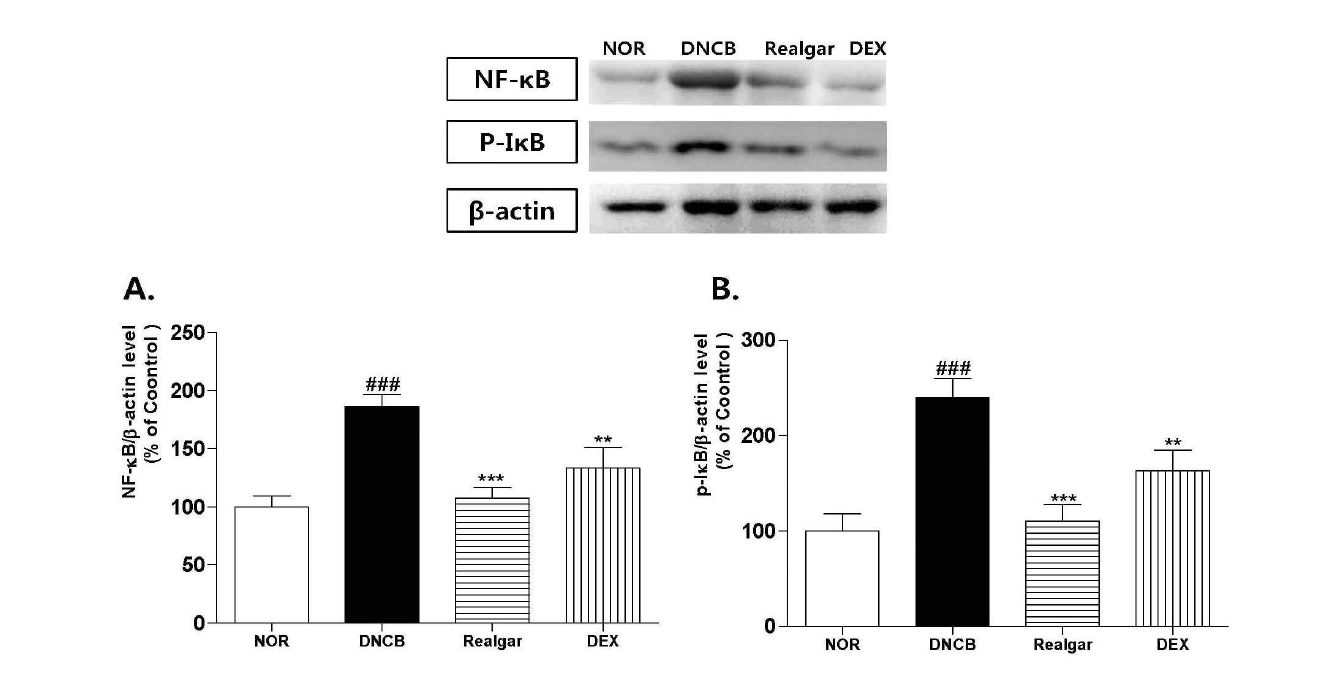
Changes in expressions of NF-κB, phospho-IκBα upon application of realgar. The effects of realgar on the expressions of NF-κB and phospho-IκBα was assessed using western blot analysis. Results shown are representative of five observations. Data are presented as means ± SD and analyzed with ANOVA. ###P < 0.001, versus the NOR group. ***P < 0.001, **P < 0.01, versus the DNCB group.
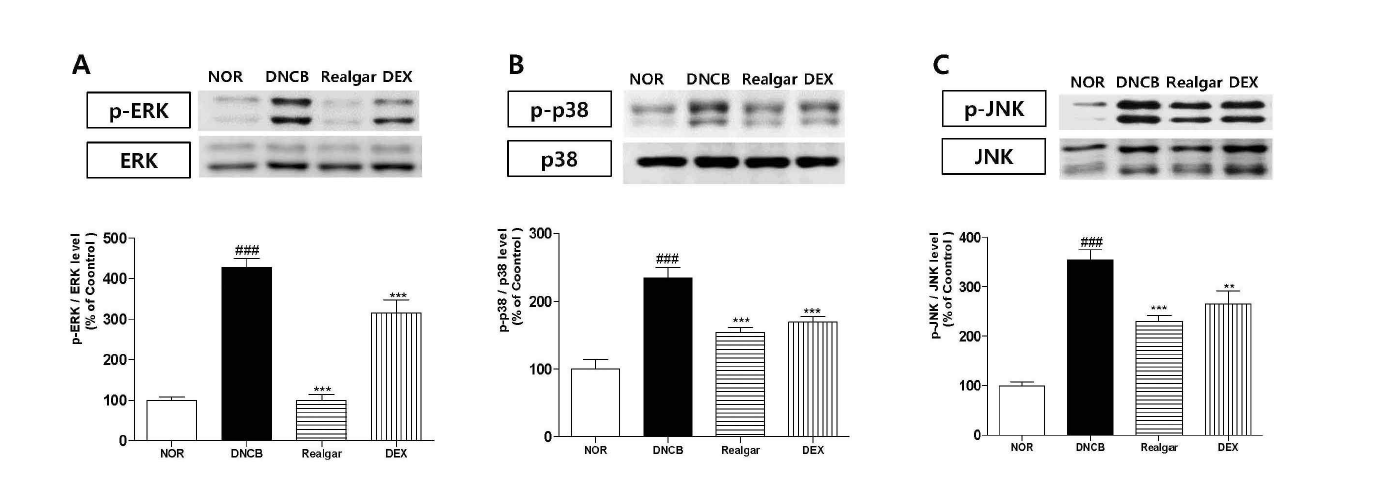
Changes in MAPKs upon application of realgar. (A) phospho-ERK1/2, (B) phospho-p38 and (C) phospho-JNK. Total protein of the dorsal skin was analyzed by western blotting. Results shown are representative of five observations. Data are presented as means ± SD and analyzed with ANOVA. ###P < 0.001, versus the NOR group. ***P < 0.001, **P < 0.01, versus the DNCB group.