Effect of Picrorrhizae Rhizoma Aqueous Extracts on Paw Chronic Inflammation In Mice
Article information
Abstract
Objectives:
The purpose of this study was to examine the efficacy of Picrorrhizae Rhizoma (PR) aqueous extracts on the formalin-induced paw chronic inflammation in mice.
Methods:
PR extracts (500, 250 and 125mg/kg) or distilled water (DW) were orally administered once a day for 10 days to formalin-injected chronic inflammatory mice. The paw thicknesses and volumes were measured daily and the paw wet-weight and histological profiles were conducted at termination with paw tumor necrosis factor (TNF)-α contents measurement. The anti-inflammatory effects of PR extracts were compared with dexamethasone.
Results:
In DW treated control group, the paw thickness, paw wet-weights and paw TNF-α contents were markedly increased. Severe chronic inflammation signs such as severe fibrosis, the formation of necrotic debris, and infiltration of inflammatory cells were detected in histopathological observations. However, these formalin-induced changes were dramatically decreased by treatment of dexamethasone and all three different dosages of PR extracts. The anti-inflammatory effects of PR at highest dose were slighter than that of dexamethasone, but it did not show any harmful effects on the body weight contrary to dexamethasone.
Conclusion:
These results suggest that PR extracts have safe and favorable effects on formalin-induced chronic inflammation.
Introduction
Inflammation is an essential protective process preserving the integrity of organisms against physical, chemical and infective insults. However, the inflammatory response to several insults frequently leads to erroneous damage to normal tissues1). Physical damage, chemical substances, micro-organisms and other agents are all possible causes of acute inflammation. The inflammatory responses to such insults consist of changes in blood flow, increased permeability of blood vessels and the subsequent escape of cells from the blood into the tissues. The changes are essentially the same regardless of the cause or its location. Chronic inflammation is an inflammatory response of prolonged duration: weeks, months, or even indefinitely, where the extended time course is provoked by the persistence of the causative stimulus for inflammation in the tissue2,3). Formalin-injected hind-paw chronic inflammatory mice were generally has been used as one of classic method to detect the efficacy of anti-inflammation. Because marked chronic inflammations were evoked by aponeurotic formalin injection, the effect of a drug would be based on observation of induced paw weight, volumes and histopathological changes, mainly paw and digit skin2,4,5,6,7). It has recently been provided evidence of a widespread role of tumor necrosis factor-α (TNF-α) in mediating hyperalgesia at different levels8), both facilitating neuronal excitability and triggering the release of other pro-inflammatory substances9,10). Therefore, TNF-α is treated as one of a key pro-inflammatory cytokines in acute and chronic inflammation models11,12). Steroids have been a popular choice for treating various inflammatory disorders; however, the potential for significant local and systemic adverse events, like skin atrophy and hypothalamic-pituitary-adrenal axis suppression, has limited their use14). Dexamethasone is well-known glucocorticoid, and it is the most widely used anti-inflammatory drugs as control drug on development of the new anti-inflammatory drugs2,3,6,14).
A traditional Korean herbal medicine, Picrorrhizae Rhizoma (PR) is a dried root and stem of Picrorrhiza kurroa, and has been used as hepatoprotective agents such as jaundice. Until now, the nitric oxide scavenging activity15), cardioprotective effect16), anti-cancer effect17,18), anti-diabetic activity19), anti-viral effect20), immunostimulatory or immunomodulatory effects21,22,23), hypolipidemic and hepatoprotective effects24,25,26) of PR extracts have been evaluated. In addition, PR extracts also showed anti-inflammatory effects after oral administration on xylene-induced acute ear inflammation27) and on 2,4-dinitrofluorobenzene-nudced contact dermatitis28) with single mouse oral dose toxicity29). Although the effect of enhancing the immune system can worsen or even induce inflammation, immunomodulators can be activates macrophages or neutrophils and can remove the cellular debris resulting from oxidative damage, thereby speeding-up the recovery of damaged tissue2,30,31). Meanwhile, an immunomodulatory agent can reduce the inflammation previously observed32). For example, nitric oxide (NO) plays an important role in inflammation, and NO synthase inhibitors can reverse several classic inflammatory symptoms33) as related to anti-oxidative effects2). Therefore, it can be postulated that PR extracts will have a favorable effect on reducing or speeding-up the recovery from local chronic inflammation induced by irritants mediated by already known its anti-inflammatory27), anti-oxidative15) and/or immunomodulatory21,22,23) effects.
In the present study, the possible anti-inflammatory effects of PR extracts, a traditional Korean herbal medicine were evaluated on the formalin-induced mice paw chronic inflammation, one of the simplest animal models for detecting chronic anti-inflammation.
Materials and Methods
1. Animals and husbandry
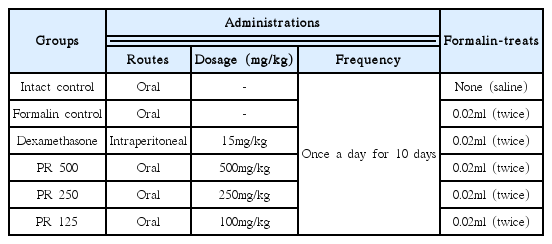
Forty-two male ICR mice (6-wk old upon receipt, SLC, Japan) were used after acclimatization for 7 days. Animals were allocated four per polycarbonate cage in a temperature (20–25ºC) and humidity (40–45%) controlled room. Light: dark cycle was 12hr: 12hr and feed (Samyang, Korea) and water were supplied free to access. All animals were fasted overnight before start of PR extracts and dexamethasone administration, and sacrifice (about 18hrs with ad libitum access to water). Total six groups, seven mice per group were used in this study as follows (Table 1). All animals were treated according to the Guide for the Care and Use of Laboratory Animals by Institute of Laboratory Animal Resources, Commission on Life Science, National Research Council, USA on 1996, Washington D.C.
2. Preparations and administration of test materials
The PR was purchased from Cho-Heung Pharmaceutical Ind. Co. (Daegu, Korea) after confirming the morphology under microscopy. The voucher specimen has been deposited at Department of Herbal Biothechnology, Daegu Haany University (voucher number: DHU083-PR). The prepared PR (103 g) was boiled in 2 L of distilled water for 2 hrs and filtrated. The filtrate was decompressed using a rotary vacuum evaporator (Lab. Camp, Korea) and lyophilized in a programmable freeze dryer (IlShin Lab., Korea). Total acquired PR extracts was 26.4g (yield 25.65%). Powders of PR extracts were stored in a refrigerator at −10ºC to protect against light and moisture. Three different dosages of PR aqueous extracts – 500, 250 and 125mg/kg were selected based on the results on the xylene-induced acute inflammation27), and 15mg/kg of intraperitoneal treatment of dexamethasone was also selected from previous report2,3,27). The PR extracts dissolved in distilled water were orally administered and dexamethasone dissolved in saline was intraperitoneally administered once a day for 10 days. For intact and formalin control mice, only vehicle (distilled water) was orally administered at a volume of 10ml/kg instead of PR extracts or dexamethasone in the present study. (Table 1)
3. Induction of chronic inflammation
One hour before start of test article administration, a subaponeurotic injection of 0.02ml of 3.75% formalin (Sigma, MO, USA) was administered to the left hind paw (planta pedis) on the first and third day of the experiment. The right hind paw was considered as the control. In the case of the intact control, the same volume of saline as that used in the other dosing groups, including the formalin control, was administered in the same region using the same method according to the previous method2).
4. Changes in body weights
Daily body weights of all experimental animals used in this study were measured from 1 day before start of test article and formalin treatment, once a day to 10 days with automatic electronic balance (Precisa Instrument, Switzland). In addition, body weight changes during 10 days of test article or vehicle treatment periods as follows:
5. Paw thickness and volume measurements
The thicknesses of the induced paw were measured using an electronic digital caliper (Mytutoyo, Japan) and recorded once a day for 10 days from 1 hr before first formalin injection. One hour before the formalin-injection or 2 hrs before test articles administration, the lengths of the long axis (longitudinal; excluding dactyl region) and short axis (cross) of induced hind paws were measured using an electronic digital caliper and recorded once a day for 10 days. The paw volume was calculated as follows according to the previous report2) :
6. Paw wet-weight measurement
At sacrifice, the wet-weight of induce paws was measured, and to reduce any deviation due to individual body weight differences, the relative weight (%) was calculated using the body weight at sacrifice and absolute weight as follows:
7. Paw TNF-α content measurement
After paw wet-weight measurements, for TNF-α evaluation, skin samples of the left induced paws were homogenized in 3 ml of phosphate-buffered saline (PBS) containing 10 mM EDTA and 20KIU/ml aprotinin (Sigma, MO, USA). After centrifugation at 10,000×g, the supernatant were frozen at −70°C for TNF-α assay as previous report11). The levels of TNF-α in paw supernatants were measured by means of an enzyme-linked immunosorbent assay (ELISA) kit specific for mouse TNF α (Bender Medsystem, Prodotti Gianni, Milano, Italy). The anti-TNF capture monoclonal antibody (mAb) (5 Ag/ml) was absorbed on a polystyrene 96-well plate and the TNF-α present in the sample was bound to the antibody-coated wells. The biotinylated anti-TNF detecting mAb (0.25 Ag/ml) was added to bind TNF-α captured by the first antibody. After washing, streptavidin-peroxidase was added to the wells to detect the biotinylated detecting antibody and finally TMB substrate was added. A colored product was formed in proportion to the amount of TNF-α present in the sample, which was measured at optical density 450 nm. The amount of cytokine in each supernatant was extrapolated from the standard curve. The standards were recombinant cytokine curves generated in doubling dilutions from 2500 to 39pg/ml.
8. Histopathology
The dorsum pedis (including the subcutaneous regions) skin were separated from the hind paws, and longitudinally trimmed. Thereafter, they were fixed in 10% neutral buffered formalin. In addition, metatarsal regions including second metatarsal bones were crossly trimmed, and fixed in 10% neutral buffered formalin. Then decalcified using decalcifying solution [24.4% formic acid, and 0.5N sodium hydroxide] for 5 days (mixed decalcifying solution was exchanges once a day for 5 days). After that, prepared dorsum pedis skin and metatarsal regions embedded in paraffin, sectioned (3∼4μm) and stained with Hematoxylin & Eosin (H&E). The histological profiles of the paws were observed ompared with that for the intact control.
Histomorphometry - The thicknesses from epidermis to hypodermis of dorsum pedis (thickness-dorsum pedis) and dorsum digit skin (thickness-dorsum digit) were measured as histomorphometrical analyses with number of infiltrated inflammatory cells on skin of dorsum pedis (IF cells-pedis) and dorsum digit (IF cells-digit) on longitudinally trimmed induced dorsum pedis skin and crossly trimmed metatarsal regions. The histopathologist was blindes to group distribution when this analysis was made.
9. Statistical analyses
All data were expressed Mean±standard deviation (SD) of seven mice, and multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test. If the Levene test indicated no significant deviations from variance homogeneity, the obtain data were analyzed by one way ANOVA test followed by least-significant differences (LSD) multi-comparison test to determine which pairs of group comparison were significantly different. In case of significant deviations from variance homogeneity were observed at Levene test, a non-parametric comparison test, Kruskal-Wallis H test was conducted. When a significant difference is observed in the Kruskal-Wallis H test, the Mann-Whitney U-Wilcoxon Rank Sum W test was conducted to determine the specific pairs of group comparison, which are significantly different. Statistical analyses were conducted using SPSS for Windows (Release 6.1.3., SPSS Inc., USA).
Results
1. Changes on the body weight
No meaningful changes on the body weights and gains were detected in formalin control as compared with intact control throughout total 10 days of experimental periods. However, dexamethasone-treated mice showed significant (p<0.01 or p<0.05) decreases on the body weights and gains were detected from 3 days after administration as compared with intact and formalin control, respectively. In addition, marked significant (p<0.01 or p<0.05) increases of the body weights and gains were detected in PR extracts 500mg/kg treated mice from 5 days after administration as compared with intact and formalin control (arrows), respectively. Anyway no meaningful changes on the body weights and gains were detected in PR extracts 250 and 125mg/kg treated mice as compared with intact control throughout total 10 days of experimental periods, respectively (Fig 1 and 2).
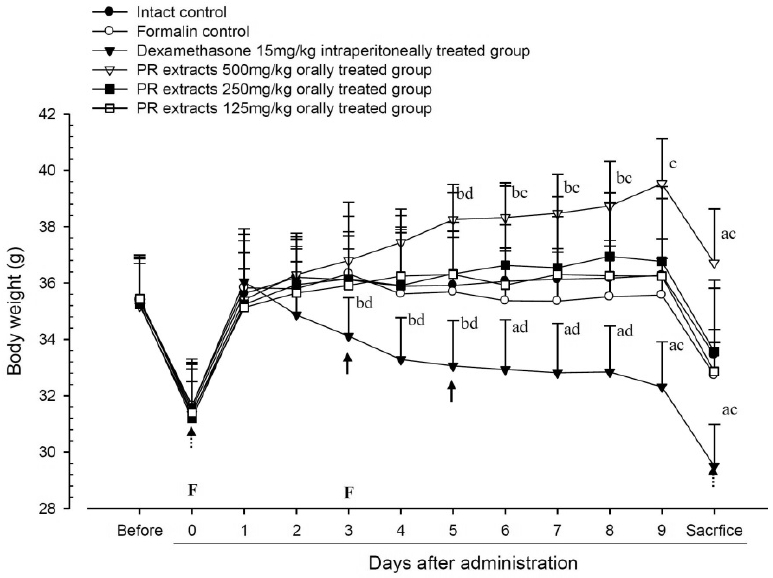
Body Weights Detected during 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; Before means 1 day before first formalin treatment or start of test material administration; Subaponeurotic injection of formalin was conducted at Day 0 and Day 3, respectively (F); All animals were overnight fasted at Day 0 and sacrifice (dot arrows); a p<0.01 and b p<0.05 as compared with intact control by ANOVA test; c p<0.01 and d p<0.05 as compared with Formalin control by ANOVA test.
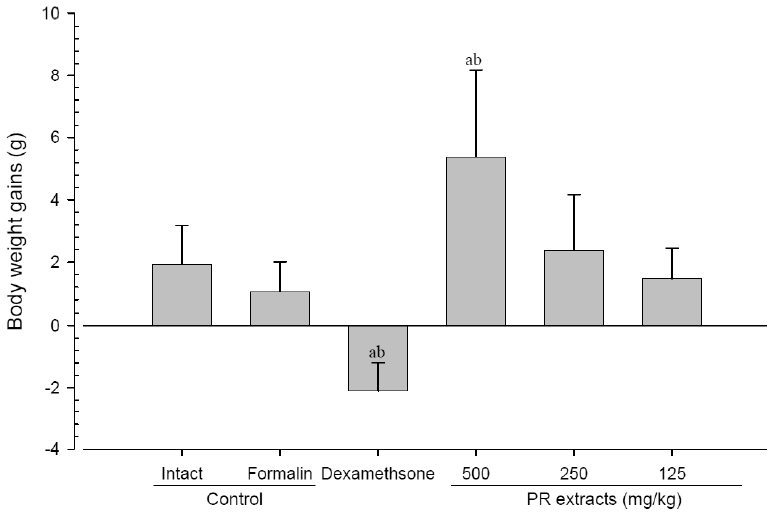
Body Weights Gains after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; a p<0.01 as compared with intact control by ANOVA test; b p<0.01 as compared with Formalin control by ANOVA test.
2. Changes in paw thickness
A significant (p<0.01) increase of induced paw thickness was detected in the formalin-injected control compared with that in the intact control from 1 day after formalin injection, accordingly the changes of paw thicknesses during 10 days of experimental periods were also significantly (p<0.01) increased in the present study. However, the paw thicknesses were significantly (p<0.01 or p<0.05) decreased compared with that in the formalin-injected control from 3 day after start of dexamethasone treatment, from 5 days after start of PR extracts 500mg/kg treatment and from 7 days after start of PR extracts 250 and 125mg/kg administration, and the changes of thicknesses were also significantly (p<0.01) decreased in dexamethasone and all three different dosages of PR extract treated mice as compared with formalin control, respectively. A clear dose-dependent decrease in the paw thickness was detected in the PR extract administered groups (Fig 3 and 4).
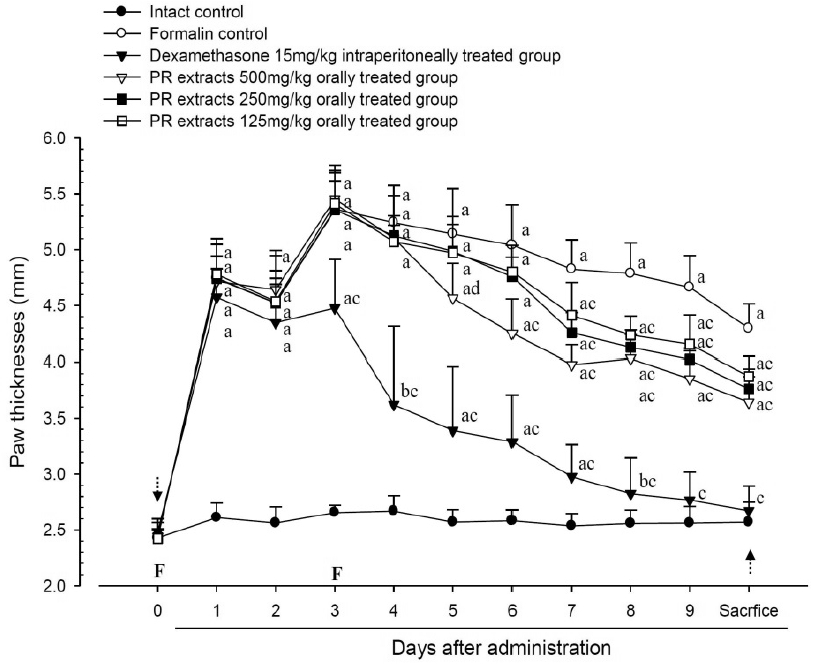
Paw Thicknesses Detected during 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; Subaponeurotic injection of formalin was conducted at Day 0 and Day 3, respectively (F); All animals were overnight fasted at Day 0 and sacrifice (dot arrows); a p<0.01 and b p<0.05 as compared with intact control by ANOVA test; c p<0.01 and d p<0.05 as compared with Formalin control by ANOVA test.
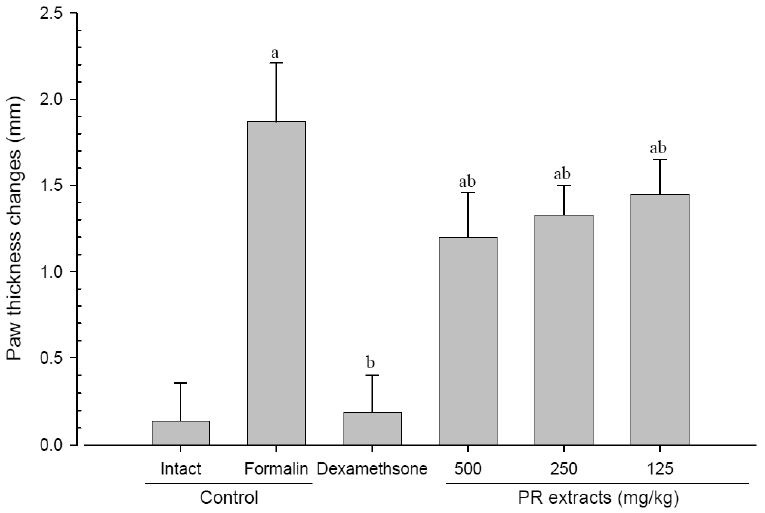
Paw Thicknesses Changes after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma, a dried root and stem of Picrorrhiza kurroa; a p<0.01 as compared with intact control by ANOVA test; b p<0.01 as compared with Formalin control by ANOVA test.
3. Changes in paw volume
A significant (p<0.01) increase of induced paw volume was detected in the formalin-injected control compared with that in the intact control from 1 day after formalin injection, accordingly the changes of paw volumes during 10 days of experimental periods were also significantly (p<0.01) increased in formalin control. However, the paw volumes were significantly (p<0.01 or p<0.05) decreased compared with that in the formalin-injected control from 3 days after start of dexamethasone, from 6 days after start of PR extracts 500mg/kg treatments and from 7 days after start of PR extracts 250 and 125mg/kg administration, and the changes of volume were also significantly (p<0.01) decreased in dexamethasone and all three different dosages of PR extract treated mice as compared with formalin control, respectively. A clear dose-dependent decrease in the paw volume was detected in the PR extract administered groups (Fig 5 and 6).
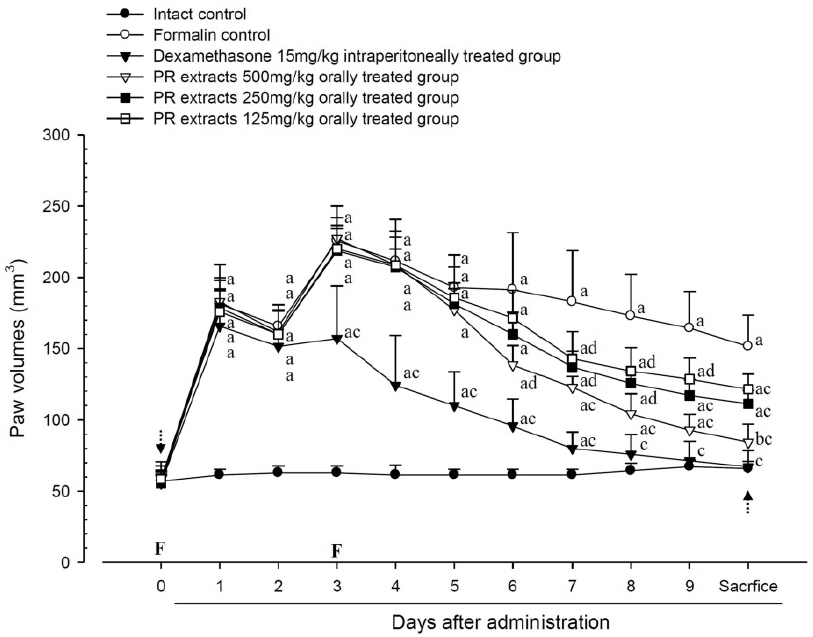
Paw Volumes Detected during 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; Subaponeurotic injection of formalin was conducted at Day 0 and Day 3, respectively (F); All animals were overnight fasted at Day 0 and sacrifice (dot arrows); a p<0.01 and b p<0.05 as compared with intact control by ANOVA test; c p<0.01 and d p<0.05 as compared with Formalin control by ANOVA test.
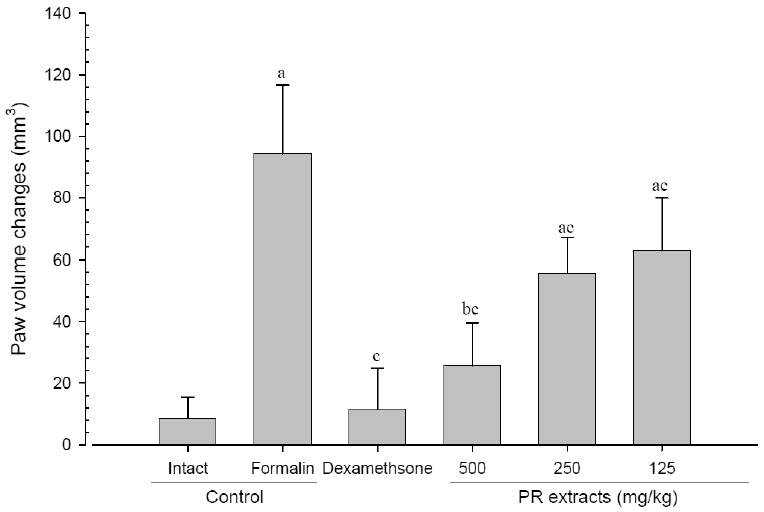
Paw Volume Changes after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; a p<0.01 and b p<0.05 as compared with intact control by ANOVA test; c p<0.01 as compared with Formalin control by ANOVA test.
4. Changes in paw weight
A significant (p<0.01) increase of induced paw absolute and relative weights was detected in the formalin-injected control compared with that in the intact control. However, the paw weights were significantly (p<0.01 or p<0.05) decreased in all administered groups compared with that in the formalin-injected, respectively. A clear dose-dependent decrease in the paw weights was detected in the PR extract administered groups (Fig 7 and 8).
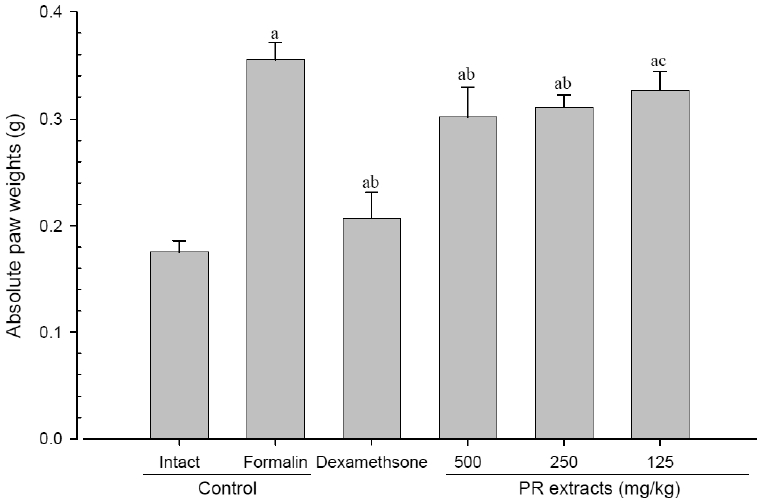
Absolute Paw Weights Detected after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; a p<0.01 as compared with intact control by ANOVA test; b p<0.01 and c p<0.05 as compared with Formalin control by ANOVA test.
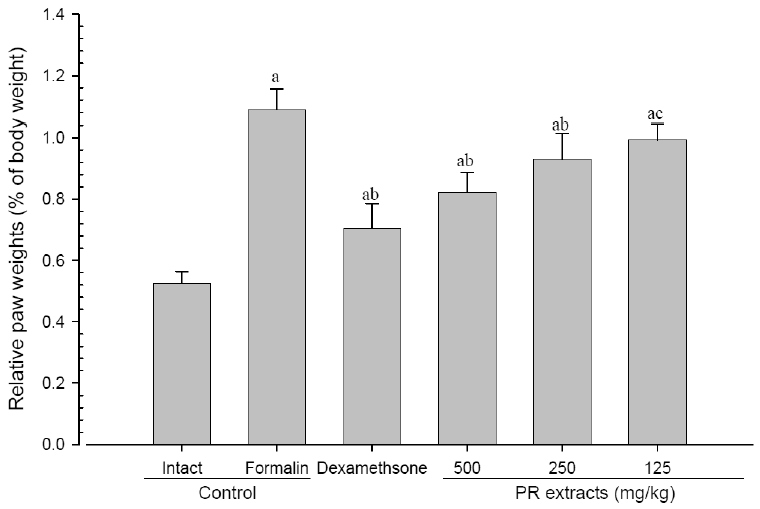
Relative Paw Weights Detected after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; a p<0.01 as compared with intact control by ANOVA test; b p<0.01 and c p<0.05 as compared with Formalin control by ANOVA test.
5. Changes in paw TNF-α contents
A significant (p<0.01) increase of induced paw TNF-α contents was detected in the formalin-injected control compared with that in the intact control. However, the paw TNF-α contents were significantly (p<0.01 or p<0.05) decreased in all administered groups compared with that in the formalin-injected, respectively. A clear dose-dependent decrease in the paw TNF-α contents was detected in the PR extract administered groups (Fig 9).
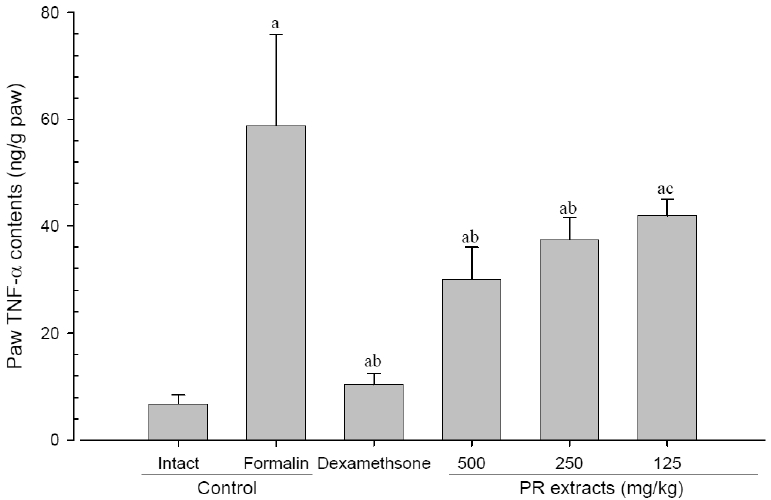
Paw TNF-α Contents Measured after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Values are expressed mean, S.D. of seven mice; PR, aqueous extracts of Picrorrhizae Rhizoma; TNF-α, Tumor necrosis factor-α; a p<0.01 as compared with intact control by ANOVA test; b p<0.01 and c p<0.01 as compared with Formalin control by ANOVA test.
6. Histopathology
Histopathological changes related to chronic inflammation, such as severe fibrosis, the formation of necrotic debris, and infiltration of inflammatory cells, were observed in the both dorsum pedis and dorsum digit skins of formalin-injected control, leading to the hypertrophy of subcutaneous regions. In all the administered groups, including the PR extract treated groups; these histopathological changes were dramatically decreased as compared with formalin-injected control. In addition, a clear dose-dependency was also demonstrated in the PR extracts treated groups (Fig 10 and 11).
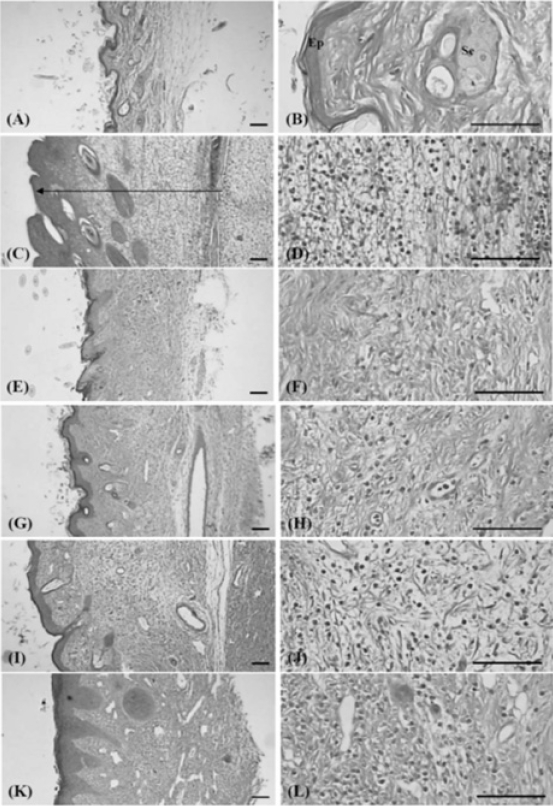
The Representative Histological Profiles of Dorsum Pedis Observed after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Intact control mice (A, B); Formalin control mice (C, D); Dexamethasone 15mg/kg treated mice (E, F); PR extracts 500mg/kg treated mice (G, H); PR extracts 250mg/kg treated mice (I, J); PR extracts 125mg/kg treated mice (K, L)
PR, aqueous extracts of Picrorrhizae Rhizoma; Arrow indicated the thicknesses of dorsum pedis skin measured; Ep, epithelium – keratinized stratified squamous epithelium; Se, sebaceous gland; All H&E stain; Scale bars = 160μm.
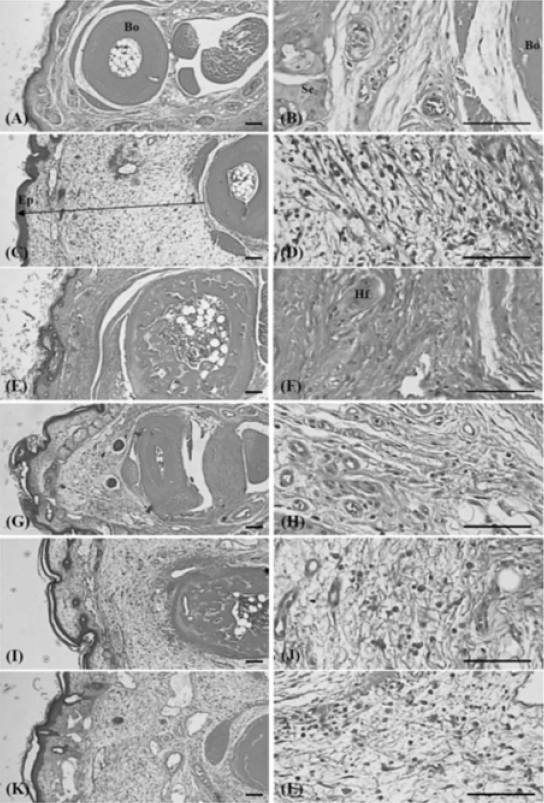
The Representative Histological Profiles of Dorsum Digit Observed after 10 Days of Continuous Oral Treatment Periods of PR extracts in Formalin-induced Chronic Inflammation Mice
Intact control mice (A, B); Formalin control mice (C, D); Dexamethasone 15mg/kg treated mice (E, F); PR extracts 500mg/kg treated mice (G, H); PR extracts 250mg/kg treated mice (I, J); PR extracts 125mg/kg treated mice (K, L)
PR, aqueous extracts of Picrorrhizae Rhizoma; Arrow indicated the thicknesses of dorsum digit skin measured; Bo, metatarsal bones; Hf, hair follicle; Ep, epithelium – keratinized stratified squamous epithelium; Se, sebaceous gland; All H&E stain; Scale bars = 160μm.
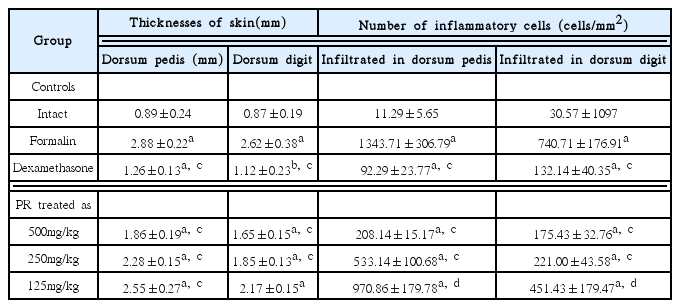
1) Changes of paw skin thicknesses
Significant (p<0.01) increases in the thickness of the dorsum pedis and dorsum digit of the induced paw were detected in the formalin control as compared with intact control. However, this increased thickness was dramatically decreased in all the dosed groups when compared with that in the formalin-injected control. In addition, a clear dose-dependency was also demonstrated in the PR extracts treated groups (Table 2).
2) Changes of the numbers of inflammatory cells infiltrated in paw skin
Significant (p<0.01) increases in the numbers of the inflammatory cells on the dorsum pedis and dorsum digit of the induced paw were detected in the formalin control as compared with intact control. However, this increased infiltrated inflammatory cells were dramatically and significantly (p<0.01 or p<0.05) inhibited by treatment of dexamethasone and all three different dosages of PR extracts, respectively as compared with formalin-injected control. In addition, a clear dose-dependency was also demonstrated in the PR extracts treated groups (Table 2).
Discussion
In the present study, the possible anti-inflammatory effects of PR extracts, a traditional Korean herbal medicine were evaluated on formalin-induced mice paw chronic inflammation, one of the simplest animal models for detecting chronic anti-inflammation2,4,5,6,7) as comparing with dexamethasone, 15mg/kg intraperitoneally treated mice.
As results of twice subaponeurotic formalin treatments, a marked increase in the paw thickness and volume was detected in the formalin-injected control as compared with that in the intact control, plus at the time of sacrifice the paw wet-weights, paw TNF-α contents were also dramatically increased. In histopathological observations, severe chronic inflammation signs such as such as severe fibrosis, the formation of necrotic debris, and infiltration of inflammatory cells, were detected in the formalin-injected control, and marked increases in the thickness of the skin of the dorsum pedis and of the dorsum digit with increases in infiltrated inflammatory cells on dorsum pedis and dorsum digits skins, respectively. However, these formalin-induced chronic inflammatory changes were dramatically decreased by treatment of dexamethasone and all three different dosages of PR extracts in the present study.
PR extracts showed anti-inflammatory effects after oral administration on acute inflammation animal models27) and on 2,4-dinitrofluorobenzene-nudced contact dermatitis28). It also has been demonstrated that anti-oxidative15) and/or immunomodulatory21,22,23) effects of PR extracts; directly related to anti-inflammatory effects2,30,31,32,33). Therefore, previously reported anti-oxidative 15) and/or immunomodulatory21,22,23) effects of PR extracts were considered one of the major mechanisms of the anti-inflammatory effects detected in the present study.
TNF-α, a 17-kDa protein which was first identified as a product of activated macrophages34), is one of well-known proinflammatory cytokines35) and they were involved in various inflammations11,12,36,37). TNF may potentiate inflammations by stimulating the release of eicosanoids and other cytokines, such as interleukin (IL)-1 and TNF-α. IL-1 activates neutrophils and macrophages, increasing the production and the release of reaction oxygen species and nitric oxide, which has been implicating in local tissue damages38). It has recently been provided evidence of a widespread role of TNF-α in mediating hyperalgesia at different levels 8), both facilitating neuronal excitability and triggering the release of other pro-inflammatory substances9,10). In the present study, PR extracts dose-dependently inhibited the elevations of paw TNF-α induced by subaponeurotic treatment of formalin.
The body weight decreases detected in dexamethasone treated group were considered as direct toxicity of glucocorticoid, steroids have been a popular choice for treating various cutaneous disorders; however, the potential for significant local and systemic adverse events, like skin atrophy and HPA axis suppression, has limited their use13). Marked increases of body weight and gains detected in PR extracts of the present study is considered as secondary changes from immunomodulatory effects already known21,22,23). Anyway, no meaningful changes on the body weight and gains were detected by treatment of PR extracts 250 and 150mg/kg as compared with formalin control in this study, and they showed body weight changes ranged in the normal aged matched mice as previously39,40).
After a local injection of formalin, marked increases in the paw thickness, volume, and weight were detected as the general chronic inflammation response, and these increases have already been used as valuable markers for testing anti-inflammatory effects5). In the present study, the increased paw thickness, volume, and weights were markedly inhibited by treatment of all three different dosages of PR extracts. Consequently, these inhibitions were considered as direct evidence that PR extracts tested in this study had a favorable effect on reducing the chronic inflammatory response.
Histopathologically, severe fibrosis, the formation of necrotic debris, infiltration of inflammatory cells, mainly lymphocytes, and hypertrophy of subcutaneous regions are used as signs of chronic inflammation after a local injection of formalin, also resulting in a marked increase in the thickness of the skin (including hypodermis) with infiltration of inflammatory cells2). However, in the present study, these histopathological changes were markedly and dose-dependently inhibited after treatment of all three different dosages of PR extracts. These inhibitions were considered as direct evidence that the PR extracts had a relatively favorable effect on reducing the chronic inflammatory response.
The results obtained in this study is considered as direct evidences that PR extracts have safe and favorable effects on formalin-induced chronic inflammation as like acute inflammation already reported.