A Study on Single Dose Toxicity of Mecasin Pharmacopuncture Injection in Muscle
Article information
Abstract
Objectives:
This study was carried out to analyze the single dose toxicity of Mecasin(Gami-Jakyak Gamcho buja Decoction) pharmacopuncture in muscle of Sprague-Dawley rats.
Methods:
All experiments were performed at the Medvill, an institution acknowledged to conduct non-clinical studies, under the Good Laboratory Practice (GLP) regulations. Sprague-Dawley rats were chosen in this pilot study. The reason Sprague-Dawley rats were chosen is that they have been widely used in safety test in the field of medicine, so the results can be easily compared with many other databases. Doses of Mecasin pharmacopuncture, 0, 500, 1,000, and 2,000mg/kg, were registered to the experimental groups, and a dose of normal saline solution, 10 ml/kg, was registered to the control group. Mecasin pharmacopuncture and normal saline were injected into the thigh of the rats by disposable syringes at intervals of six hours twice a day. This study was performed under the approval of the Institutional Animal Ethic Committee.
Results:
There is no death or abnormality in any of the four groups. No significant changes in weight, hematological parameters or clinical chemistry between the control group and the experimental groups were observed. To inspect abnormalities in organs and tissues, we used microscopy to examine representative histological sections of each specified organ; the results showed no significant differences in any of the organs or tissues.
Conclusion:
The above outcomes suggest that treatment with Mecasin pharmacopuncture is relatively safe. Further evaluations and studies on this subject are needed to prove more concrete evidence.
Introduction
Mecasin(Gami-Jakyak Gamcho buja Decoction) pharmacopuncture was developed for treating amyotrophic lateral sclerosis patient with pain, joint contracture and muscular weakness.[1] Amyotrophic lateral sclerosis(ALS) is a progressive and incurable disease that causes degeneration of the motor neurons of the brain stem and the spinal cord[2]. About half of ALS patients reported pain; described as nagging, annoying, boring and exhausting[3].
The constituents of Mecasin pharmacopuncture are Paeonia Radix, Glycyrrhizae Radix, Aconiti Lateralis Preparta Radix, Curcuma longa, Gastrodiae Elata, Radix Salivae Miltriorrhizae, Atratylodes japonica, Chaenomelis fructus, Polygala tenuifolia Willd. There have been several studies conducted about those compositions of toxicity and effectiveness. Nevertheless, toxicity testing of Mecasin acupuncture has not been conducted yet. Therefore, this study was performed to analyze the single-dose toxicity and lethal dose of Mecasin acupuncture in rats.
To study acute and subacute toxicity through Good Laboratory Practice(GLP) regulations is the current research trend for single-dose toxicity testing of extracts. All the experiments for this study were conducted at Medvill.
Materials and Methods
The Mecasin pharmacopuncture was prepared in a sterile room at the Medvill. Paeonia Radix, Glycyrrhizae Radix, Aconiti Lateralis Preparta Radix, Curcuma longa, Gastrodiae Elata, Radix Salivae Miltriorrhizae, Atratylodes japonica, Chaenomelis fructus, Polygala tenuifolia Willd. were mixed, pulverized and extracted in 30% ethanol (KP) for 3h at 84∼90°C, concentrated by a rotary evaporator and lyophilized. The entire procedure was repeated two times. After the mixing process with water for injection, the Mecasin pharmacopuncture concentration was controlled to 1000:1. Additionally water for injection was added to make a normality. The completed extract was stored in a refrigerator (2.5∼5.9°C).
The animals used in this study were 7-week-old Sprague-Dawley rats. The reason Sprague-Dawley rats were chosen is that they have been widely used in safety test in the field of medicine, so the results can be easily compared with many other databases. The mean weights of the rats were 306.1 −335.2g and 227.3 −255.7g, respectively, for the male and the female rats at the time of Mecasin pharmacopuncture injection. For all animals, a visual inspection was conducted; all animals were weighed at the beginning. During 10 days of acclimatization, the general symptoms of the rats were observed once a day. The weights of the rats were recorded on the last day of acclimatization. No abnormalities were found.
The temperature of the lab was 19.8 – 29.7°C, and the humidity was 47.4% – 76.8%. Enough food (Lab Diet 5053) and UV-filtered water were provided.
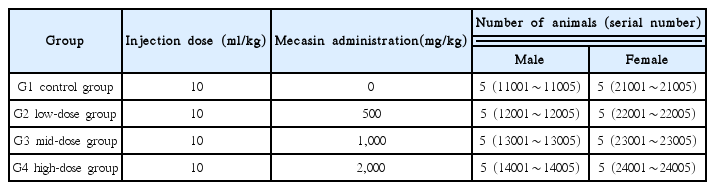
Groupings were done after acclimatization period. Animals were selected when their weights were close to the mean weight. Finally, 20 male rats and 20 female rats were selected in total. The animals were randomly distributed into 4 groups (5 male and 5 female rats per group) as shown in Table 1.
In this study, 2,000 mg/kg was set as a high-dose, and 1,000 mg/kg and 500 mg/kg were set as mid and low doses, respectively. In the control group, 10 ml/kg of normal saline solution was administered. Injection dose was same for all group(10ml/kg). Mecasin pharmacopuncture and normal saline were injected into the thigh of the rats by disposable syringes at intervals of six hours twice a day. This study was conducted under the approval of the Institutional Animal Ethic Committee of Medvill Co., Ltd.
From the 1st day to 14th day after treatment, the general symptoms were examined once a day. On the day of dosing (day 0), the general symptoms (side effects, revealing time, recovery time, etc.), as well as mortality, were examined at 30 min and at 1, 2, 4, and 6 hours after injection. The weights were measured immediately before treatment and at 1, 3, 7 and 14 days after injection. After observations had been finished, we conducted necropsies of the rats after cutting these abdominal aorta and vein under anesthetization by using CO2. The organs of all surviving animals were visually inspected.
The weight data from the experiments were analyzed by using SPSS program (SPSS 16.0). A Levene test was conducted to evaluate the homogeneity of the variance and the significance. The one-way ANOVA test was conducted when the homogeneity of the variance was recognized. And the Kruskal-Wallis's H-test was conducted when the homogeneity of the variance was not recognized. A significance level of P<0.05 was used throughout.
Results
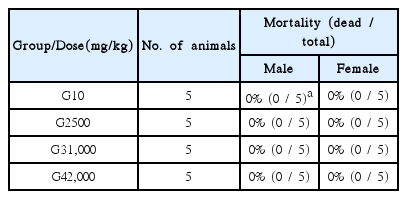
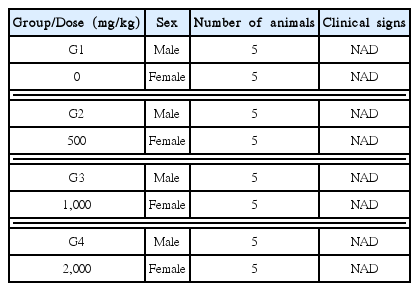
In this study, no deaths or abnormalities occurred in any of the groups (Tables 2,3).
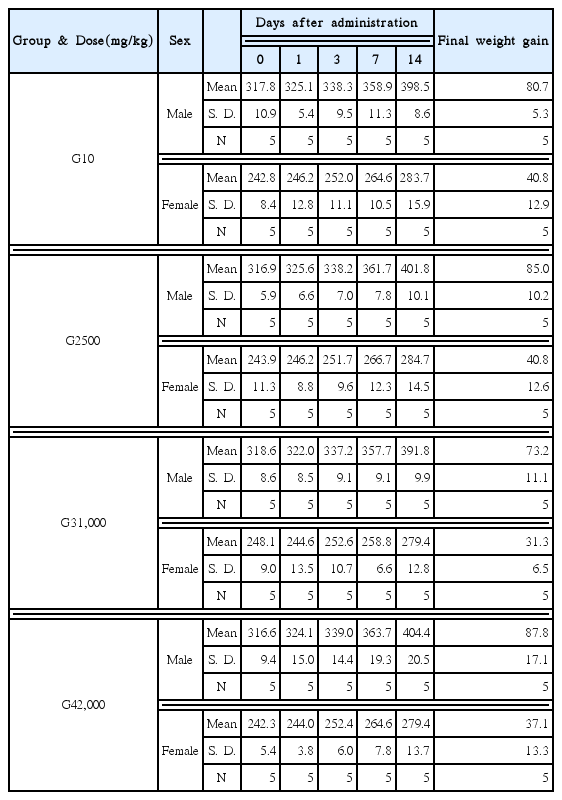
In weight examinations, slight decreases in the weights of some rats were observed. Weights of one male rat in the control group, one male rat in the 2,000 mg/kg injection group, one female rat in the control group, one female rat in the 500 mg/kg injection group, three female rats in the 1,000 mg/kg group, one female rat in the 2,000 mg/kg group decreased temporarily at 1 day after injection. At 3 days after injection, weights of one female rat in the control group, one female rat in the 500 mg/kg group, one female rat in the 1,000 mg/kg group decreased temporarily and recovered. At 7 days after injection, weights of one female rat in the 1,000mg/kg injection group decreased temporarily. But increases in the weight were observed in the other rats (Table 4).
In both control and experimental groups, no meaningful changes in the necropsy were noted (Table 5).
Discussion
Mecasin pharmacopuncture has been utilized in clinics to relieve pain, joint contracture and muscular weakness especially for amyotrophic lateral sclerosis patients[1]. A recent study showed that Jakyak Gamcho Decoction (JGT) and its constituents have a protective effect against tert-butyl hydroperoxide (t-BHP)-induced cytotoxicity in the hippocampal HT22 cell line [4]. In addition, Jakyak Gamcho buja Decoction, which consists of JGT and Radix Aconiti Lateralis Preparata, is believed to be useful for suppressing the progress of osteoarthritis because of its anti-inflammatory effects and its ability to reduce pain with histopathological efficacy [5]. Because of these facts, Mecasin has been studied for treating patients with joint pain, muscle spasms, and arthralgia due to cold[1]. But, No clinical review on the effects of Mecasin pharmacopuncture is published. However, there have been many studies on the component herbs of this pharmacopuncture.
Paeonia Radix aqua-acupuncture solution(PRAS) has antioxidative effects. Antioxidant effects of PRAS on lipid peroxidation were determined by TBA method and also by ammonium thiocyanate method during the autoxidation of linoleic acid. PRAS exhibited markedly antioxidant activity, which inhibited 82% of linoleic acid peroxidation. And PRAS showed 48% scavenging effect on α,α-diphenyl-β-picrylhydrazyl (DPPH) radical. PRAS protected the cell death induced by tert-butyl hydroperoxide(t-BHP) and significantly increased cell viability in the normal rat liver cell(Ac2F). These results suggested that PRAS might play a protective role in lipid peroxidation by free radicals.[6]
Glycyrrhizae Radix extract should be beneficial in the inhibition of allergic inflammatory response.[7]
Jakyak Gamcho Tang-herbal acupuncture, which consists of Paeonia Radix and Glycyrrhizae Radix, has a analgesic effect and could be used safely. Cheon YS et al. showed that the Jakyak Gamcho Tang-herbal acupuncture has more significant analgesic effect than the control group. And they reported that there was not an example of expired mouse for 2weeks after this experiment.[8]
Aconiti Lateralis Preparta Radix aqua-acupuncture has the analgesic and anti-imflamatory effect on the arthritis induced by Freund`s complete Adjuvant in rats. Jeoong SH et al. measured the levels of plasma serotonin, WBC, RBC, Hb, ESR after injecting Aconiti Lateralis Preparta Radix aqua-acupuncture. In this experiment, the levels of plasma serotonin, WBC, RBC, Hb, and ESR are decreased and the paw edema was also decreased[9]. The Aqua-acupuncture with Radix aconiti at an early stage can bring about better movement recovery in patients with spinal cord injuries[10]. Furthermore, it is a relatively safe treatment[11].
Curcuma longa LINNE pharmacopuncture at ST36 has a suppressing inflammation effect on Freund's adjuvant arthritis in rats[12].
Gastrodiae Elata pharmacopuncture at GB20 showed anti-apoptotic and neuroprotective effects on cholinergic neuronsin focal cerebral ischemia caused by stroke in SD rats[13].
Aqua acupuncture of Radix Salivae Miltriorrhizae water extracts may modulate renal function by increasing the glomerular filtration rate in normal rats[14].
Atratylodes japonica-herbal acupuncture in ST40 SP9 is effective on body weight, food efficiency ratio, the level of serum lipid, protection of liver function and prevention of cardiovascular risk by obesity induced by high fat diet[15].
Chaenomelis fructus herbal acupuncture could be effective in the treatment of muscle atrophy[16].
Polygala tenuifolia Willd. pharmacopuncture study has not conducted yet, but Polygala tenuifolia Willd. is used widely as an anti-inflammatory, sedative that is thought to exert a variety of neuropsychiatric effects[17].
Although Mecasin has been used in clinics, safety studies on Mecasin are insufficient, so more safety studies are needed. Toxicity tests provide important data and are essential for evaluating the safety of test substances in medications [18]. This study was performed to provide objective safety data for Mecasin. Doses of 500 mg/kg, 1,000 mg/kg, and 2,000 mg/kg of Mecasin were injected to the experimental groups, and a dose of 10 ml/kg of normal saline solution was administered to the control group. In all four groups, no deaths occurred, and no abnormalities were found. No significant differences in the clinical signs or weights were noted between the control group and the experimental groups. In necropsy for checking for abnormalities in organs, no significant findings were noted.
To assess the oral toxicity of Mecasin, we need to study its acute and chronic side effects more. We also need more study on hematologic examinations and blood chemistry tests. Animal testing is the most fundamental and basic way to perform safety assessments [19]. The Korea Food & Drug Administration has testing protocol guidelines for the study of toxicity, and all experiments should be conducted following GLP regulations [20]. The results of our study showed that injection of 2,000 mg/kg of Mecasin did not cause any changes in the weights of SD rats or in the results of necropsy examinations. It also did not result in any mortality, which indicates that Mecasin administration can be used as a safe treatment.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute(KHIDI), funded by the Ministry of Health & welfare, Republic of Korea(grant number : HI11C2142).