Kaurenoic acid, a Diterpene Derived from Aralia continentalis, Alleviates Lipogenesis in HepG2 Cells.
Article information
Abstract
Objectives
Here we investigated the anti-lipogenic potential of kaurenoic acid (KA), a diterpene derived from Aralia continentalis, in a cellular model of non-alcoholic fatty liver disease.
Methods
HepG2 cells were treated with palmitate for 24h to induce intracellular lipid accumulation. To assess the influence of KA on steatotic HepG2 cells, various concentration of KA was co-administered. After palmitate treatment, Intracellular triglyceride content was measured. Expression level of several lipogenic genes, sterol regulatory element-binding transcription factor-1c (SREBP-1c) , acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD-1) were measured using Western-blot analyses or RT-PCR.
Results
Palmitate markedly increased intracellular triglyceride level and expression of related lipogenic genes in HepG2 cells, and which was relieved by co-administered KA.
Conclusions
It is conceivable that that KA may have a pharmacological potential to reduce lipid accumulation in non-alcoholic fatty liver disease.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a condition defined by excessive accumulation of triglycerides within hepatocytes without excessive alcohol intake1). NAFLD includes a spectrum of disease from simple steatosis, steatohepatitis to fibrosis, which can progress to irreversible cirrhosis and even hepatocellular carcinoma2). NAFLD is now considered the hepatic manifestation of the metabolic syndrome, and gradually becoming the most common cause of chronic liver disease, along with the increase of obesity world widely3).
To find effective therapeutic agent, numerous pharmacologic approaches have been examined, including insulin sensitizing agents, lipid lowering agents, antioxidants, and etc4). However, there is no proven therapeutic agent as of now5).
In this study, we tried to test anti-lipogenic potential of kaurenoic acid, a natural compound derived from root of Aralia continentalis, in a steatosis cellular model induced with palmitate in HepG2 cells. Through this study we got to know that KA alleviates intra cellular lipid accumulation by preventing the induction of lipogenic genes, which is essential to NAFLD. Our findings suggest that this natural compound can be applied as a potential therapeutics for NAFLD.
Material and Methods
1. Reagents
Kaurenoic acid, 3-(4,5-dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). SREBP-1 antibody, Lamin A/C antibody, goat anti-rabbit IgG-HRP, and rabbit anti-goat IgG-HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). EnzyChrom triglyceride assay kit was purchased from BioAssay Systems (Hayward, CA, USA)
2. Cell culture
HepG2 cells originated from human hepatocellular carcinoma cell were purchased from American Type Culture Collection (Rockville, MD, USA). and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 100 U/ml penicillin, 100 μg/ml streptomycin and 10 % (v/v) heat-inactivated fetal bovine serum, and maintained in a incubator set at 37 °C with 5 % CO2 prior to experiment.
3. MTT assay
MTT assay was done to evaluate the cytotoxicity of KA on HepG2 cells. After cells were treated with KA for 24 h in a 96 well plate at the density of 1.0 ×104 cells/well, MTT solution (2.0 mg/ml) was added to each well. At 4 h after incubation at 37 °C in a humidified incubator with 5% CO2, formazan crystals formed in viable cells were dissolved with DMSO and the optical density (OD) was measured at 540nm with a microplate reader. Cell viability was calculated as a percentage against the untreated. All experiments were performed three times independently.
4. Intracellular lipid content assessment
HepG2 cells were treated with palmitate 0.5 mM for 24h to induce intracellular lipid accumulation with various concentration of KA (0, 10−9, 10−8, 10−7, and 10−6 M). HepG2 cells were trypsinized and transferred into a 1.5 mL Eppendorf tube and spindowned at 956 ×g(rcf) for 5 min. Cell pellets were washed once with PBS, resuspended in 400 μL PBS buffer and transferred to a micro smashing tube for ultrasonication. After ultrasonication, the concentration of cellular triglyceride was determined using an EnzyChrom triglyceride assay kit according to the protocol provided by the manufacturer.
5. Semi-quantitative RT-PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and reverse-transcribed using M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) to obtain cDNA. The cDNA was amplified by PCR using TaqPCRx DNA Polymerase (Invitrogen, Carlsbad, CA, USA) with a set of each primer. The PCR conditions were an initial denaturation at 95 °C for 5 min followed by 22–30 cycles of denaturation at 95 °C for 40 sec, annealing at 57 °C for 40 sec and extension at 72 °C for 50 sec with a final extension at 72 °C for 7 min. Amplicons were separated in 1.2% agarose gels in 1×TBE buffer at 100 V for 30 min, stained with ethidium bromide, and visualized under UV light. GAPDH (Glyceraldehyde -3-phosphate dehydrogenase) was used as internal controls to evaluate relative expressions of SREBP-1c, ACC, FAS, and SCD-1. Relative expression of each gene over GAPDH was determined by densitometric analysis software ImageJ (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA).
Specific primer of each gene was as follows: the forward and the reverse primers for human SREBP-1c were 5’-CAGTGGAGGGAACACAGACG-3’ and 5’-AAAGACTGGGCTGTCAGGCT-3’, respectively; human ACC were 5’-GGAACAGTGTGCGGTGAAAC-3’ and 5’-TCACTAGTGATCCGAGCAGC-3’, respectively; human FAS were 5’-GACATCGTCCATTCGTTTGTG-3’ and 5’-GTTGACATTGTACTCGGCGG-3’, respectively; human SCD-1 were 5’-GCCCCTCTACTTGGAAGACG-3’ and 5’-CGAGCTTTGTAAGAGCGGTG-3’, respectively; human GAPDH were 5’-AAGGGTCATCATCTCTGCCC-3’ and 5’-GTGATGGCATGGACTGTGGT-3’, respectively.
6. Western blot analysis
Total cell lysate was prepared with radioimmuno- precipitation assay (RIPA) buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 1% sodium orthovanadate, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS) containing protease inhibitor cocktail (Roche, Indianapolis, IN, USA), and nuclear proteins were isolated by NE-PER nuclear extraction kit (Thermo Scientific, Schaumburg, IL, USA) according to the manufacture’s instruction. The amounts of proteins in each extract were quantitated by Bradford assay. Equal amounts of proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto Polyvinylidene fluoride (PVDF) membrane using an electroblotting apparatus. After blocked with 5% non-fat milkin Tris-buffered saline (TBS) at room temperature for 1 h, the blots were incubated with SREBP-1c and lamin A/C polyclonal antibodies at 4 °C overnight and subsequently with appropriate secondary antibodies at room temperature for 1 h. The bands of interest were visualized with chemiluminescence detection kit (SuperSignal® West Femto, Thermo Scientific).
7. Statistical analysis
Student’s t-test and one-way analysis of variance (ANOVA) tests with Tukey’s post hoc test were applied for comparison of the means (with PASW Statistics Data Editor v23 Korean, SPSS Inc, Chicago, IL, USA), and P values less than 0.05 are considered statistically significant.
Results
1. Determination of the influence of KA on cell viability in HepG2 cells
To determine a safe dose range of KA without cellular toxicity, HepG2 cells were treated with different concentrations of KA, from 10−9 to 10−5 M for 24h, and the cell viability was measured by MTT assay. As shown in Figure 1, KA did not cause any significant cellular toxicity to the concentration 10−5 M, the maximal dose tested in this study.
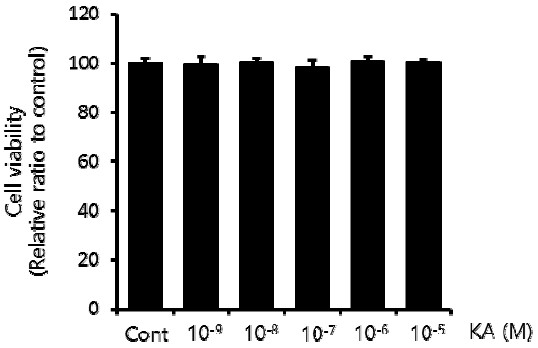
Influence of KA on Cell Viability in HepG2 Cells.
HepG2 cells was incubated with various concentration of KA from 10−9 to 10−5 M for 24h. in sequence, MTT assay was done according to the protocol described in above material and method section. Cell viability was expressed as a percentage to the KA untreated control. Data are presented as mean±SEM (n=3).
2. Establishment of the steatosis model in HepG2 Cells and the effect of KA on Intracellular Lipid Content
First, we established our cellular steatosis model by incubating HepG2 cells in a medium containing 0.5 mM palmitate and 1% bovine serum albumin. To assess the effect of KA on intraceullar lipid accumulation, various concentration of KA (10−9, 10−8, 10−7, and 10−6 M) was co-administered with palmitate. Triglyceride assay indicated increased intracellular triglyceride content in HepG2 cells treated with 0.5 mM palmitate for 24 h. The palmitate induced lipid accumulation was significantly suppressed by KA, when the concentration of KA was 10−8 M or higher (Figure 2).
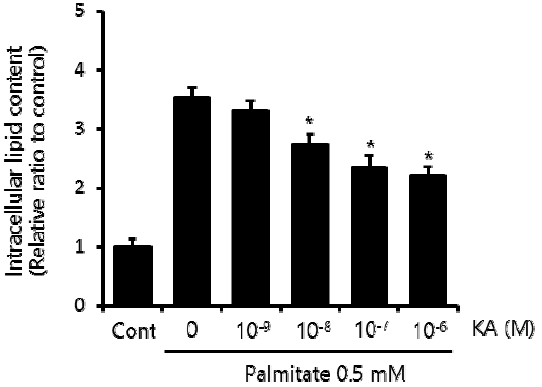
Effect of KA on Intracellular Lipid Content in Steatotic HepG2 Cells.
To induce steatosis, HepG2 cells were treated with palmitate (0.5 mM). To assess the effect of KA on intraceullar lipid accumulation, various concentration of KA (10−9, 10−8, 10−7, and 10−6 M) was co-administered. After 24h incubation, Intracellular triglyceride content was measured according to the protocol described in above material and method section. Data are caculated as a relative ratio to control, and presented as mean±SEM (n=3). The * indicates P<0.05.
3. The effect of KA on expression of lipogenic genes in palmitate treated HepG2 cells
SREBP-1c is the major regulator of lipogenesis in the liver6). Here we measured the effect of KA on palmitate induced SREBP-1c expression in HepG2 cells. Palmitate (0.5 mM, 24 h) markedly increased the mRNA level of SREBP-1c (Figure 3A), and concomitant treatment with KA (10−8, 10−7 M) significantly decreased this induction. Similar inhibitory effect of KA was also observed in the protein level of SREBP-1c (Figure 3B). SREBP-1c increases hepatic lipid accumulation by enhancing a series of lipogenic enzymes, including ACC, FAS and SCD-17). So we measured the effect of KA on this lipogenic enzymes in the second place. KA co-treatment also significantly decreased ACC, FAS and SCD-1 mRNA level elevated by fatty acid overload in HepG2 cells (Figure 4).
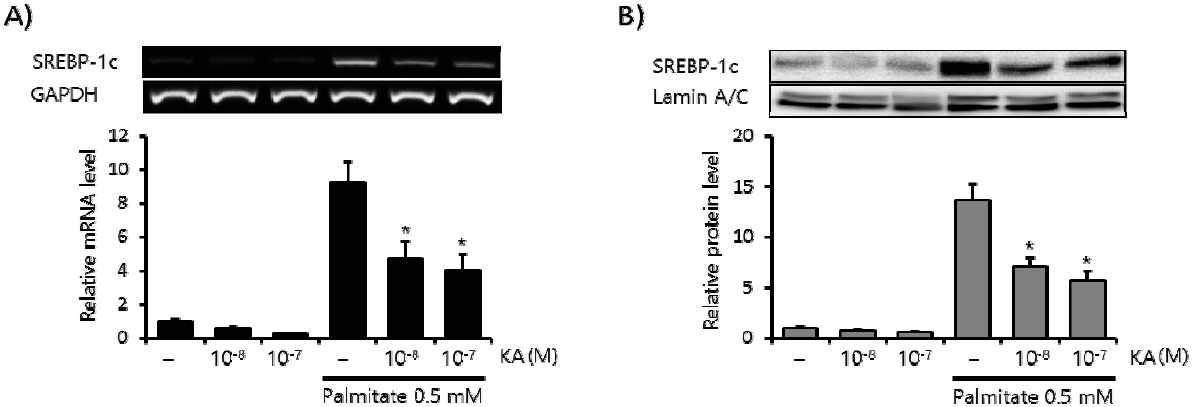
Effect of KA on Expression of SREBP-1c in HepG2 Cells.
The effect of KA on the expression of SREBP-1c by PCR (A) and Western blot analysis (B). The relative intensity of each band was calculated over GAPDH or Lamin A/C. Data are presented as the mean±SEM (n=3). The * indicates P<0.05, compared to just palmitate treated cells without KA (the 4th bar of each graph).

Effect of KA on Lipogenic Genes, ACC, FAS, and SCD-1 in HepG2 Cells.
Expression levels of lipogenic genes were assessed with semi-quantitative RT-PCR (A), and relative mRNA levels of ACC (B), FAS (C), and SCD-1 (D) were expressed on each graph. Data are caculated as a relative ratio, and presented as mean±SEM (n=3). The * indicates P<0.05, compared to just palmitate treated cells without KA (the 4th bar of each graph).
Discussion
Kaurenoic Acid (KA) (Figure 5) is one of the natural compound extracted from root of Aralia continentalis Kitagawa, which is one of the most popular traditional herbal medicine in Korea, called ‘Dokhwal’8). Root of Aralia continentalis was traditionally used to reduce pain or inflammation, such as headache, arthralgia, toothache, common colds, and various kinds of skin infection9). Most of known pharmacological potentials of KA were also related to such effect, especially inflammation or infection8,10–12).
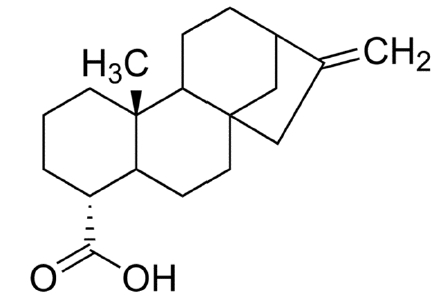
Chemical Structure of Kaurenoic Acid (KA).
Empirical Formula C20H30O2. Molecular Weight 302.45. Kaurenoic acid a diterpene derived from root of Aralia continentalis, a popular herbal medicine in Korea.
In classic view of pathology, inflammation and steatosis is quite different condition. However, in recent studies some regulatory factor of inflammation, such as Nrf2, also related to hepatic steatosis13,14). Hence, we tried to test a new pharmacological activity of KA in steatosis, although KA and Aralia continentalis have never been reported to be effective in this disease elsewhere.
We examined the influence of KA on intracellular lipid content and SREBP-1c pathway, which plays a critical role in the regulation of hepatic lipid metabolism through transcriptional activation of lipogenic genes, including ACC, FAS, and SCD-115). KA significantly suppressed transcription of ACC, FAS, and SCD-1, by down-regulating SREBP-1c induction in palmitate treated HepG2 cells. As a result, KA markedly reduced intracellular triglyceride concentration. Taken together, KA seems to suppress hepatic lipid accumulation by inhibiting SREBP-1c pathway mediated hepatic lipogenesis.
Conclusions
Here we showed that KA prevented hepatic steatosis in palmitate induced NAFLD cellular model, and the anti-lipogenic effect of KA is associated with suppression of a lipogenic transcription factor SREBP-1c. Based on this results, we propose a potential usage of KA in the treatment of NAFLD, which warrants additional studies.
Acknowledgements
This work was supported by a 2-Year Research Grant of Pusan National University.