The Effects of Lycii Radicis Cortex on Inflammatory Response through an Oxidative Stress and AGEs-mediated Pathway in STZ-induced Diabetic Rats
Article information
Abstract
Objectives
This study examined whether Lycii Radicis Cortex has an inhibitory effect on inflammatory response through an oxidative stress and advanced glycation endproducts (AGEs)-mediated pathway in streptozotocin (STZ)-induced type 1 diabetic rats.
Methods
Lycii Radicis Cortex was orally administered to STZ-induced diabetic rats in doses of 80 or 160 mg/kg body weight/day for 2 weeks, and its effects were compared with those of diabetic control and normal rats.
Results
The administration of Lycii Radicis Cortex decreased the elevated serum urea nitrogen and renal reactive oxygen species (ROS), and reduced the increased AGEs in the serum and kidney. The elevated protein expressions of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits in the kidney of diabetic control rats were significantly decreased after Lycii Radicis Cortex treatments. Moreover, the kidney of diabetic rats exhibited the up-regulation of receptor for AGEs (RAGE) and AGEs-related proteins; however, Lycii Radicis Cortex treatment also significantly reduced those expressions (excepted RAGE). In addition, the diabetic rats exhibited an up-regulation of the expression of proteins related to inflammation in the kidney, but Lycii Radicis Cortex administration reduced significantly the expression of the inflammatory proteins through the nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) pathways.
Conclusions
This study provides scientific evidence that Lycii Radicis Cortex exerts the antidiabetic effect by inhibiting the expressions of AGEs and NF-κB in the STZ-induced diabetic rats.
Introduction
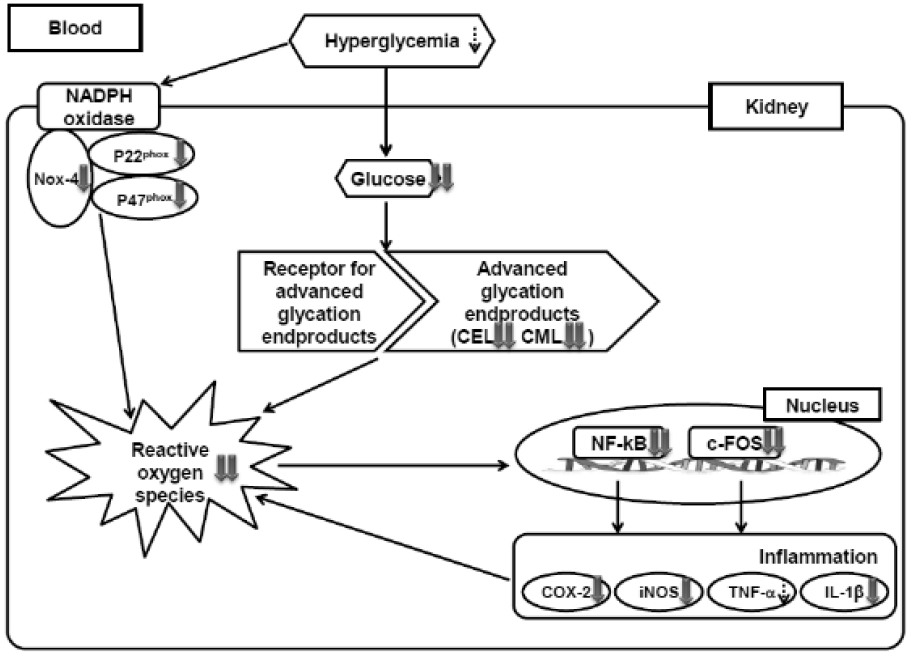
Diabetes mellitus is a metabolic disease characterized by prolonged hyperglycemia that can lead to micro and macrovascular diseases. Especially the microvascular injury in diabetes mainly targets three major organs, the eye, peripheral nerve, and kidney1,2). Diabetic nephropathy is the most common cause of end-stage renal disease, and could account for disability and high mortality rate in patients with diabetes. The pathogenesis of diabetic nephropathy is multifactorial in which long-term hyperglycemia plays a crucial role2). During diabetic milieu, supraphysiological glucose is involved in the formations of advanced glycation endproducts (AGEs) and the mitochondrial productions of free radicals, and leading consequently to cell death and renal dysfunction. Therefore, oxidative stress plays a pivotal role for the development of diabetic nephropathy, which is characterized by the thickening of glomerular basement membranes, expansion of mesangial cells, glomerular hypertrophy, loss of podocytes, expansion of tubular basement membranes, tubular atrophy, interstitial fibrosis, and arteriosclerosis3).
AGEs and nonenzymatic glycation of proteins contribute to diabetic tissue injury. The accumulation of AGEs in kidney and other tissues of patients with diabetes mellitus has been implicated in the development of diabetic nephropathy and vasculopathy4,5). AGEs may contribute to diabetic tissue injury by at least two major mechanisms. The first is receptor -independent alteration of the extracellular matrix architecture by nonenzymatic glycation and the formation of protein crosslinks. The second mechanism is receptor-dependent and consists of modulation of cellular functions through ligation of specific cell surface receptors, the best characterized of which is the receptor for AGEs (RAGE)6,7). AGEs-RAGE binding, which activates nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, is a central player in the production of superoxide radicals8). AGEs have been shown to induce oxidative stress and to activate two major pro-inflammatory transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1)9).
The root bark of Lycium chinense Miller, Lycii Radicis Cortex, extract has been widely used as a traditional Korean medicinal herb for centuries. Lycii Radicis Cortex is sweet in taste and cold in nature and effectively works for the kidney, lung and liver channels. The medicinal plant has been extensively applied for diabetes mellitus with the traditional functions of cooling the blood, bringing down hectic fever, clearing away heat, and removing fire in the lung10). Pharmacological studies in animal models or cell lines revealed that extracts from Lycii Radicis Cortex are able to lower blood pressure11), serum glucose12), lipid levels13), and to improve insulin resistance14). Our previous study has shown that the Lycii Radicis Cortex extract reduces oxidative stress and its related renal damage in cisplatin-induced nephrotoxicity rats15). In addition, it has been proved that the major compound of Lycii Radicis Cortex is plenty of phenols, which have favorable effects of protection against inflammation, atherosclerosis, cancer, and diabetes in association with oxidative stress 16–19). Therefore, in this study, we examined the effect of Lycii Radicis Cortex on the oxidative stress- and reactive oxygen species (ROS)-related factors involved in the development of diabetic damage using streptozotocin (STZ)-induced type 1 diabetic rats.
Materials and Methods
1. Materials
The protease inhibitor mixture, ethylenediaminetetraacetic acid (EDTA) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 2′,7′-Dichlorofluorescein diacetate (DCFH-DA) was obtained from Molecular Probes (Eugene, OR, USA). The Bio-Rad protein assay kit and pure nitrocellulose membrane were supplied by Bio-Rad Laboratories (Seoul, Korea). Phenylmethylsulfonyl fluoride (PMSF) was acquired from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit polyclonal antibodies against RAGE, NAD(P)H oxidase-4 (Nox-4), p22 phagocytic oxidase (p22phox), p47phox, NF-κBp65, and mouse monoclonal antibodies against cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), β-actin, histone and goat polyclonal antibodies against tumor necrosis factor-a (TNF-a) and inteleukin-1β (IL-1β) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit polyclonal anti-AP-1 gene c-FOS were obtained from Cell Signaling Technology, Inc. (Cell Signaling, MA, USA). Monochlonalanti-Ne-(carboxyethyl) lysine (CEL) antibody, and polyclonal anti-Ne-(carboxymethyl) lysine (CML) antibody were kindly provided by Dr. R. Nagai (Kumamoto University, Japan). Goat anti-rabbit and goat anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated secondary antibodies were acquired from Santa Cruz Biotechnology, Inc. ECL Western Blotting Detection Reagents were supplied by GE Healthcare (Piscataway, NJ, USA).
2. Preparation of Lycii Radicis Cortex Extracts
Lycii Radicis Cortex, collected in China, was purchased from Human-herb (Gyeongsan, Korea). To prepare the Lycii Radicis Cortex extract, the roots (150 g) of Lycii Radicis Cortex were boiled gently in 2 L of water for 3 h. After filtration, the solution was evaporated under reduced pressure and then freeze dried to provide an extract with a yield of 10% by weight of the starting materials. A voucher specimen of Lycii Radicis Cortex has been deposited at the herbarium located at the College of Korean Medicine, Daegu Haany University.
3. Experimental Animals and Treatment
Animal experiments were performed according to the “Guidelines for Animal Experimentation” approved by Daegu Haany University. Six-week-old male Sprague-Dawley rats were purchased from Daehan-Bio (Chungcheong, Korea). The rats were maintained under a 12-h light/dark cycle, and housed in a controlled temperature (22 ± 2°C) and humidity (40 ± 5%) environment. After several days of adaptation, the rats were randomly separated into normal control (n=6) and diabetic groups. The diabetic groups were injected intraperitoneally with STZ (Sigma-Aldrich, St. Louis, MO, USA) (50 mg/kg body weight) in 10 mM citrate buffer (pH 4.5). After 3 days of STZ injection, the glucose levels of blood taken from the tail veins were measured, and then the STZ-induced diabetic rats were divided into three groups. Treatment with Lycii Radicis Cortex was initiated after confirming the induction of hyperglycemia in the diabetic rats by weight (197.3 ± 0.2 g) and serum glucose (399.0 ± 0.5 mg/dl). The diabetic control group (n=6) was given water orally, while the other two groups (n=6 per group) were orally administered with Lycii Radicis Cortex extracts daily for 14 days at a dose of 80 or 160 mg/kg body weight, respectively. The non-diabetic rats (n=6) as the normal group were compared with the diabetic groups. Body weight, food intake and water intake were determined every day during the experimental period. After administration for 14 days, blood samples were collected from the abdominal aorta of anaesthetized rats. Serum was separated immediately by centrifugation. Subsequently, each rat was perfused with ice-cold physiological saline, and then the kidney was harvested, snap-frozen in liquid nitrogen and stored at −80°C until analysis were performed.
4. Analysis of the Serum Parameters
The serum glucose was measured using a commercial kit (Glucose CII-Test from Wako Pure Chemical Industries, Ltd., Osaka, Japan). The renal functional parameter (serum urea nitrogen) was measured using a commercial kit (BUN Kainos from Kainos Laboratory Inc., Tokyo, Japan). The serum ROS level was determined using the method reported by Ali et al20). Serum glycosylated protein was colorimetrically measured by determining 5-hydroxymethyl furfural formation from glucose according to the thiobarbituric acid assay21).
5. Measurement of Renal Glucose and AGEs Levels
The renal glucose level was determined by the method of Momose et al.22), with some modifications. Renal tissues were homogenized with ice-cold 0.9% NaCl buffer, and then the homogenate was deproteinized by centrifugation at 1,670 × g for 15 min at 4°C, and then the glucose level was determined using the Wako kit after incubation for 30 min at 37°C. The renal AGEs level was determined by the method of Nakayama et al.23). Minced kidney tissue was delipidated with chloroform and methanol (2:1, v/v) overnight. After washing with methanol and distilled water, the tissue was homogenized in 0.1 N NaOH, followed by centrifugation at 8,000 × g for 15 min at 4°C. The amounts of AGEs in these alkali-soluble samples were determined by measuring the fluorescence at an emission wavelength of 440 nm and an excitation wavelength of 370 nm. A native bovine serum albumin (BSA) preparation (1 mg/ml of 0.1 N NaOH) was used as a standard and the fluorescence intensity values of the samples were measured at a protein concentration of 1 mg/ml and expressed in arbitrary units (AU) compared with the native BSA preparation.
6. Measurement of Renal ROS Generation Level
ROS generation was measured using the method reported by Ali et al.20) Renal tissues were homogenized on ice with 1 mM EDTA-50 mM sodium phosphate buffer (pH 7.4), and 25 mM DCFH-DA was then added to the homogenates. After incubation for 30 min, the changes in fluorescence were determined at an excitation and emission wavelength of 486 nm and 530 nm, respectively.
7. Preparation of Nuclear and Post-Nuclear Fractions
Nuclear protein extraction was performed using the method reported by Komatsu24). Briefly, renal tissues were homogenized with ice-cold lysis buffer containing 5 mM Tris-HCl (pH 7.5), 2 mM MgCl2, 15 mM CaCl2 and 1.5 M sucrose, followed by the addition of a 0.1 M dithiothreitol (DTT) and a protease inhibitor mixture. After centrifugation (10,500 × g for 20 min at 4°C), the pellet was suspended with an extraction buffer containing 20 mM 2-[4-(2-hydroxyethyl)-1-piperazyl] ethanesulfonic acid (pH 7.9), 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA and 25% (v/v) glycerol, followed by the addition of a 0.1 M DTT and protease inhibitor mixture. The mixture was placed on ice for 30 min. The nuclear fraction was prepared by centrifugation at 20,500 × g for 5 min at 4°C.
The post-nuclear fraction was extracted from the kidneys of each rat. Briefly, the renal tissue was homogenized with ice-cold lysis buffer (pH 7.4) containing 137 mM NaCl, 20 mM Tris–HCl, 1% Tween 20, 10% glycerol, 1 mM PMSF, and a protease inhibitor mixture. The homogenate was then centrifuged at 2,000 × g for 10 min at 4°C. The protein concentration in each fraction was determined using a Bio-Rad protein kit (Bio-Rad Laboratories, Hercules, CA, USA).
8. Western blot Analyses
To determine NF-κBp65, AP-1, and histone, 10 mg of protein from each nuclear fraction underwent 8% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to a nitrocellulose membrane, blocked with a 5% (w/v) skim milk solution for 1 h, and incubated separately with the primary antibodies to NF-κBp65, AP-1, and histone overnight at 4°C. After washing the blots, they were incubated with the anti-rabbit or anti-mouse IgG HRP-conjugated secondary antibody for 1 h at room temperature. In addition, 10 mg of protein of each post-nuclear fraction of RAGE, CML, CEL, Nox-4, p22phox, p47phox, COX-2, iNOS, IL-1β, TNF-a, and β-actin was electrophoresed through 8–15% SDS-PAGE. Each antigen-antibody complex was visualized using ECL Western Blotting Detection Reagents and detected by chemiluminescence with SENSI-Q2000 Chemodic (Lugen Sci. co., Ltd, Seoul, Korea). The band densities were determined using ATTO Densitograph Software (ATTO Corporation, Tokyo, Japan), and quantified as a ratio to β-actin or histone. The protein levels of the groups are expressed relative to those of the normal rats (represented as 1).
9. Statistical Analysis
Data were expressed as the mean ± SD. Statistical comparisons were performed using one-way analysis of the variance (ANOVA) followed by a Dunnett’s test (SPSS 11.5.1 for Windows, 2002; SPSS Inc., Chicago, IL, USA). The p values < 0.05 were considered significant.
Results
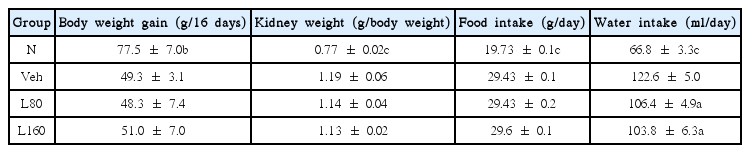
1. Body weight, kidney weight, food intake, and water intake
Table 1 shows the changes in body weight, kidney weight, food intake, and water intake during the experimental period. The diabetic control rats showed a significant decrease in body weight gain. On the other hand, the kidney weight and the food and water intakes in diabetic control rats were markedly higher than those of in normal rats. Compared to the diabetic control rats, the administration of Lycii Radicis Cortex significantly decreased the water intake at both the 80 and 160 mg/kg body weight/day, but the body and kidney weight and food intake were not changed throughout the experimental period.
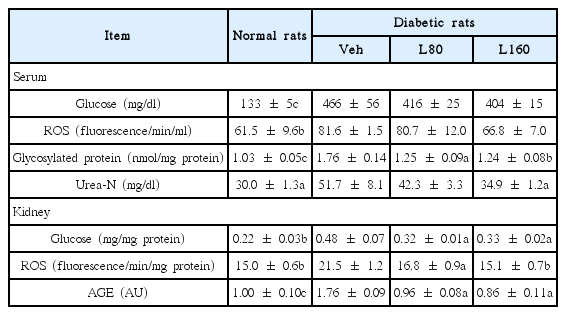
2. Biochemical analysis
As shown in Table 2, the serum and kidney glucose levels were significantly increased in diabetic control rats. Lycii Radicis Cortex administration significantly reduced the glucose levels at both the 80 and 160 mg/kg body weight/day in kidney, but it led to a tendency towards a slight decrease without significance in serum. The oxidative stress-related biomarker, the ROS, in the serum and kidney of diabetic control rats was higher than those in the normal rats. The administration of Lycii Radicis Cortex decreased the serum ROS level, but there was no statistical significance. However, in contrast in kidney, the Lycii Radicis Cortex treatment significantly decreased the ROS level in diabetic rats. In addition, the serum and kidney levels of AGEs in diabetic control rats were significantly higher than normal rats. However, Lycii Radicis Cortex treatment showed significant reduction in a dose-dependent manner in both serum and kidney. Urea nitrogen, a renal functional parameter, was markedly increased in diabetic control rats compared to normal rats, but it was significantly attenuated by the administration of Lycii Radicis Cortex at a dose of 160 mg/kg body weight/day.
3. Renal NADPH-oxidase protein expressions
The expression level of the p47phox protein is significantly elevated in the kidney of the diabetic control rats compared to the normal rats (Fig. 1C). However, the levels of NOX-4 and p22phox expressions were not changed significantly in the diabetic control rats (Fig. 1A and 1B). On the other hand, the administration of Lycii Radicis Cortex significantly reduced NOX-4, p22phox, and p47phox protein expressions at a dose of 160 mg/kg body weight/day.
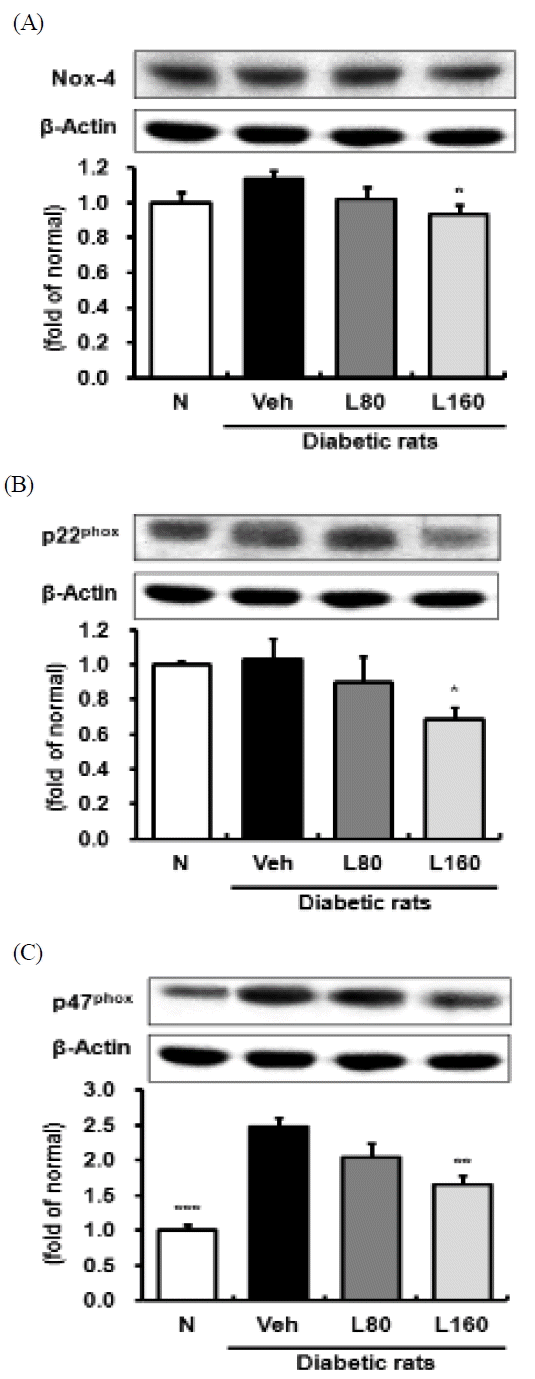
Renal NADPH oxidase subunits protein expressions
Representative immunoblots for (A) NOX-4, (B) p22phox, and (C) p47phox. Immunoblotting analysis was performed as described in Materials and methods. N: normal rats, Veh: vehicle-treated diabetic rats, L80: Lycii Radicis Cortex 80 mg/kg body weight-treated diabetic rats, L160: Lycii Radicis Cortex 160 mg/kg body weight -treated diabetic rats. Data are the mean±SD. Significance: *p<0.05, **p<0.01, ***p<0.001 versus vehicle-treated diabetic rats.
4. AGEs-related protein expressions in the kidney
Since the administration of Lycii Radicis Cortex reduced glucose levels in the kidney, we further investigated the expression of AGEs-related proteins such as RAGE, CEL, and CML by employing Western blot analysis. As shown in Fig. 2, renal RAGE, CEL, and CML were significantly elevated in diabetic control rats. Lycii Radicis Cortex-treated rats showed a significant down-regulation of CEL and CML in a dose-dependent manner (Fig. 2B and 2C). The expression levels of RAGE were reduced slightly by Lycii Radicis Cortex without significance (Fig. 2A).
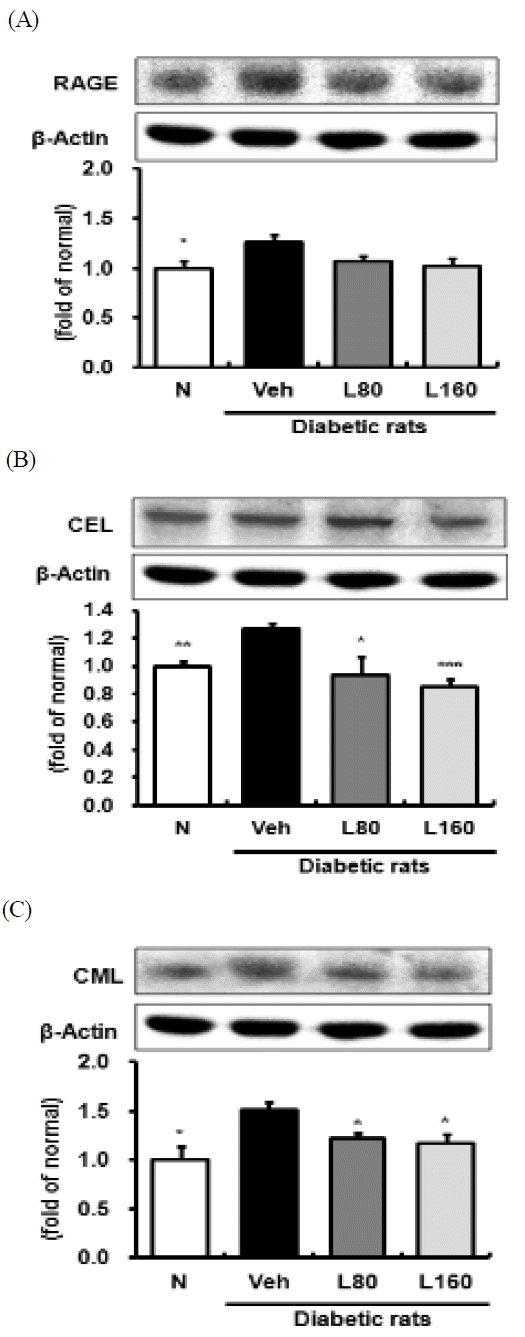
Renal receptor for advanced glycation endproducts, CEL, and CML protein expressions.
Representative immunoblots for (A) RAGE, (B) CEL, and (C) CML. Immunoblotting analysis was performed as described in Materials and methods. N: normal rats, Veh: vehicle-treated diabetic rats, L80: Lycii Radicis Cortex 80 mg/kg body weight-treated diabetic rats, L160: Lycii Radicis Cortex 160 mg/kg body weight-treated diabetic rats. Data are the mean±SD. Significance: *p<0.05, **p< 0.01, ***p<0.001 versus vehicle-treated diabetic rats.
Discussion
Recently, traditional oriental herbal medicines have been the subject of focus due to their beneficial effects observed during clinical experience accumulated over a long time and their absence of toxic or side-effects. There have thus been many studies to identify effective therapeutic agents from traditional medicines for diabetes and complications. Therefore, in this study, we investigated the effect of Lycii Radicis Cortex on the oxidative stress and ROS related factors involved in the development of diabetic renal damage using STZ-induced type 1 diabetic rats.
Insulin resistance or insulin secretion deficiency in diabetes mellitus leads to physic-metabolic abnormalities such as a decline in body weight gain and increases in food intake, water intake, urine volume, and kidney weight25). In this study, our diabetic models showed same conditions. The administration of Lycii Radicis Cortex for 14 days led to no significant difference in body and kidney weight and food intake; however, water intake was significantly reduced by both the 80 and 160 mg/kg administrations (Table 1). These results suggest that the oral administration of Lycii Radicis Cortex may improve the typical diabetic symptom, an excessive intake of water.
We observed a significant elevation of serum and renal glucose levels in diabetic rats compared with non-diabetic rats. The serum glucose levels of Lycii Radicis Cortex-treated rats was slightly lower than those of diabetic control rats; however, the concentration of renal glucose was significantly reduced by both the 80 and 160 mg/kg administrations (Table 2). It seems these results are related to that Lycii Radicis Cortex has been used as a traditional Korean herbal remedy for kidney for centuries26). Furthermore, renal functional parameters, such as urea nitrogen levels, were increased in the serum of the diabetic control group; however, urea nitrogen was significantly lowered by Lycii Radicis Cortex treatment (Table 2). These results show that Lycii Radicis Cortex treatment may ameliorate diabetic pathological conditions induced by hyperglycaemia, and may lead to improvement in the impaired renal function of diabetic rats.
Hyperglycemia is a major cause for the enhanced generation of ROS, leading to increased oxidative stress in an impaired antioxidant defense state27). ROS are considered to play a direct, central role in the pathophysiological mechanisms underlying development of various diabetic complications, because they directly oxidize and damage various tissue biomolecules, such as DNA, protein, and lipids, consequently resulting in cell dysfunction and apoptosis28,29). In the present study, diabetic rats exhibited a significant increase in levels of serum and renal ROS. On the other hand, Lycii Radicis Cortex treatment significantly reduced renal ROS in a dose-dependent manner, and showed a tendency for decrease without significance in serum (Table 2). NADPH oxidase, a major source of ROS, has been reported to be increased in experimental models of diabetic nephropathy, and importantly, the inhibition of NADPH oxidase leads to a reduction in the renal ROS production and amelioration in the morphological changes and functional abnormalities that are seen under hyperglycemia30). In addition, NADPH oxidase has been suggested to mediate generation of ROS during AGEs and RAGE interactions31). In our experiment, the diabetic control rats showed a significant up-regulated expression level of p47phox, a subunit of NADPH oxidase, compared to that in the normal rats. However, Lycii Radicis Cortex significantly reduced the p47phox level in the 160 mg/kg-treated rats (Fig. 1C). NOX-4 and p22phox were slightly increased in diabetic control rats without significance, but Lycii Radicis Cortex-treated rats showed a significant reduction of those expression levels in the 160 mg/kg-administered rats (Fig. 1A and 1B). Consequently, these results demonstrated that Lycii Radicis Cortex effectively attenuated oxidative stress, at least in part, through the direct inhibition of ROS and NADPH oxidase.
The elevated levels of glucose starts forming covalent adducts with plasma proteins through a non-enzymatic process known as glycation, and in the last stage, stable glycation products, AGEs, are formed32). AGEs are removed and metabolized by the kidney, but the kidney is also a site for the accumulation of AGEs and AGEs-associated damage33). AGEs provoke the synthesis of fibronectin, laminin, and typeIV collagen in the kidney, promoting glomerular sclerosis, interstitial fibrosis, and hypertrophy34). Additionally, the interaction of AGEs with the RAGE triggers ROS generation, NADPH oxidase and adhesion molecules expressions, and upregulates inflammation through NF-κB and other signaling pathways35,36). Therefore, the accumulation of AGEs in the kidney has been regarded as an indicator of progressive renal damage in diabetic complications. Actually, in the present study, the vehicle-treated diabetic rats showed significantly elevated levels of AGEs in serum and kidney and also, over-expressions of renal RAGE. However, the administration of Lycii Radicis Cortex significantly reduced the increased formation of AGEs in both serum and kidney (Table 2). There was only a tendency towards a decrease in the RAGE expression level from the treatment with Lycii Radicis Cortex (Fig. 2A).
One of the major AGEs, CML is generated through several pathways, such as oxidative cleavage of Amadori product by the hydroxyl radical, peroxynitrite, and also glyoxal which is generated via autoxidation of glucose37–39). Since CML formation requires oxidation in all pathways, CML is considered to be the important biomarker of oxidative stress40). In addition, CEL is reported to be generated during the reaction of methylglyoxal with lysine residues and is detected in human lens proteins at a concentration similar to that of CML. Accumulation of those AGEs increases with age and diabetes mellitus1,41). In the present study, the diabetic control rats showed enhanced protein expressions of renal CML and CEL. However, the administration of Lycii Radicis Cortex significantly down-regulated renal CEL and CML protein expression levels in a dose-dependent manner (Fig. 2B and 2C). These results suggest that Lycii Radicis Cortex can prevent diabetic renal damage through inhibiting AGEs formation, rather than AGEs-RAGE binding.
The pathobiological processes modulated by hyperglycemia and oxidative stress include inflammatory responses42). NF-κB plays a central role in oxidative stress-induced inflammatory responses by the regulation of transcription and the expression of a large number of genes, including growth factors, pro-inflammatory cytokines and others43). In particular, the activation of NF-κ B induces the inflammatory enzymes, such as COX-2 and iNOS, and subsequent production of prostaglandin E2 and nitric oxide, respectively44). AP-1, a heterodimer complex, which is typically composed of c-FOS and c-Jun, is another major redox-sensitive transcriptional factor that may be relevant to the biology of diabetic nephropathy45,46). In this study, elevated activations of NF-κB and AP-1 in the kidneys of the diabetic control rats were significantly down-regulated by Lycii Radicis Cortex administration in a dose-dependent manner (Fig. 3A and 3B). In addition, the administration of Lycii Radicis Cortex significantly decreased the protein expression level of renal COX-2 in the 160 mg/kg-treated rats, compared to that in diabetic control rats (Fig. 4A). Concerning iNOS, there was no significant difference between normal and diabetic control rats, although, Lycii Radicis Cortex treatment showed a significant decrease at a dose of 160 mg/kg body weight/day (Fig. 4B). Renal TNF-a and IL-1β expression levels were significantly increased in diabetic control rats, compared to those in the normal rats. However, the administration of Lycii Radicis Cortex significantly attenuated the level of renal IL-1β expressions in the 160 mg/kg-treated rats (Fig. 4D). Regarding the protein expressions level of TNF-a, Lycii Radicis Cortex treatment showed a reduced tendency, but it was not significant (Fig. 4C). On the basis of these results, we suggested that Lycii Radicis Cortex can adjust inflammation by inhibiting the NF-κB or AP-1 pathway in the diabetic kidney.
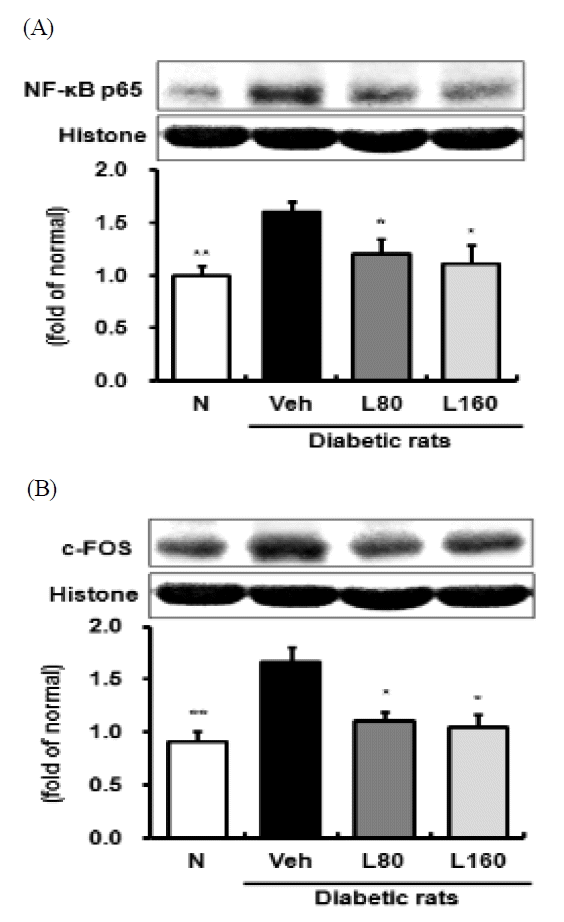
Levels of renal NF-κBp65 and c-FOS activity.
Representative immunoblots for (A) NF-κBp65 and (B) AP-1. Immunoblotting analysis was performed as described in Materials and methods. N: normal rats, Veh: vehicle-treated diabetic rats, L80: Lycii Radicis Cortex 80 mg/kg body weight-treated diabetic rats, L160: Lycii Radicis Cortex 160 mg/kg body weight-treated diabetic rats. Data are the mean±SD. Significance: *p<0.05, **p<0.01 versus vehicle-treated diabetic rats.
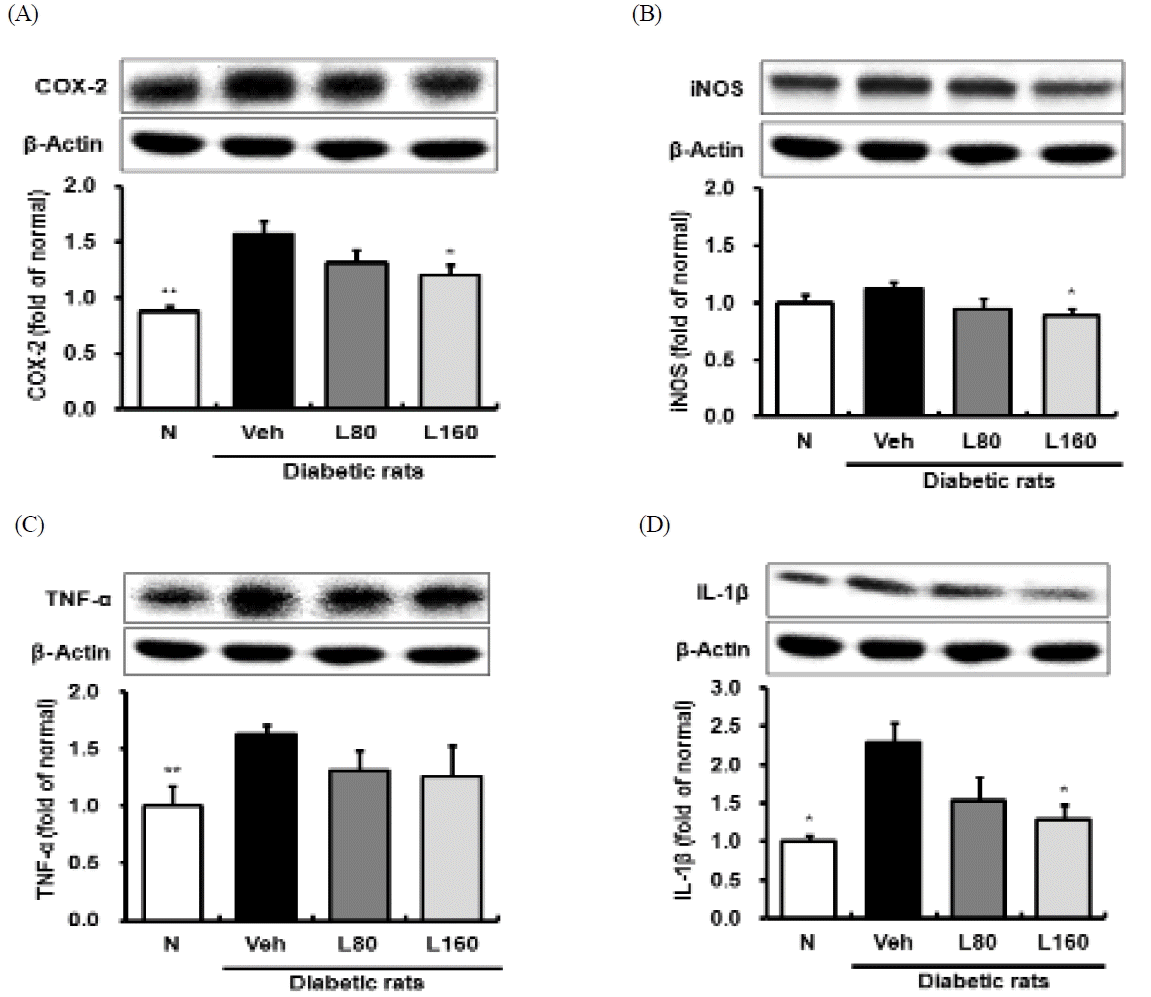
Renal inflammation-related protein expressions.
Representative immunoblots for (A) COX-2, (B) iNOS, (C) TNF-a, and (D) IL-1β. Immunoblotting analysis was performed as described in Materials and methods. N: normal rats, Veh: vehicle-treated diabetic rats, L80: Lycii Radicis Cortex 80 mg/kg body weight-treated diabetic rats, L160: Lycii Radicis Cortex 160 mg/kg body weight-treated diabetic rats. Data are the mean±SD. Significance: *p<0.05, **p<0.01 versus vehicle-treated diabetic rats.
Discussion
In conclusion, STZ-induced diabetic rats showed increased renal damage associated with the hyperglycemia-mediated oxidative stress and AGEs formation-derived pro-inflammatory transcription factors (NF-κB and AP-1), and pro-inflammatory genes (COX-2, iNOS, TNF-a, IL-1β). On the other hand, these unfavorable outcomes were reversed by Lycii Radicis Cortex administration in the kidney of diabetic rats. Lycii Radicis Cortex treatment of diabetic rats improved the overall renal function, that is confirmed through serum urea nitrogen level. Therefore, the present study suggests that Lycii Radicis Cortex is expected to provide a novel therapeutic strategy for the development of diabetic complications and further biological investigation is needed.