References
1. World Health Organization. Reducing Risks -Promoting healthy life. World Health Report 2002 Geneva: WHO; 2002.
2. Ministry of Health and Welfare. Korea Centers for Disease Control and Prevention. Korea Health Statistics 2014 Korea National Health and Nutrition Examination Survey (KNHANES VI-2).
3. Kopelman P. Health risks associated with overweight and obesity. Obesity Reviews 2007;8(1):13–17.
4. Kopelman PG. Obesity as a medical problem. Nature 2000;404(6778):635–643.
5. Lee KW. Correct Obesity Drug Therapy. Korean Journal of International Medicine 2009;77(4):971–976.
6. Lee TH. Treatment of Obesity. Korean Journal of Medicine 2001;61(3):227–233.
7. Song YK, Lim HH. Clinical Application of Ma Huang in the Obesity Treatment. Journal of Society of Korean Medicine for Obesity Research 2007;7(1):1–7.
8. Hwang MJ, Shin HD, Song MY. Literature review of herbal medicines on treatment of obesity since 2000-Mainly about Ephedra herba. Journal of Society of Korean Medicine for Obesity Research 2007;7(1):39–54.
9. Jeong JW, Cho SW. Trend research of the human body-Oriented obesity studies on Korean medicine. Journal of Korean Medicine Rehabilitation 2016;26(1):49–61.
10. Lim SY, Park SW, Joo JH, Kim SR, Kim DJ, Choi WS. The effects of Taeeumjowui-tang (Taiyintiaowei-tang) on obesity in sasang constitution. The Journal of East-West Medicine 2012;37(4):109–116.
11. Professors of formula study of Korean Medicine School. Clinical Traditional Herbalogy Seoul: Yeong-lim Press; 2016. p. 236.
12. Andraws R, Chawla P, Brown DL. Cardiovascular effects of ephedra alkaloids: a comprehensive review. Progress in Cardiovascular Diseases 2005;47(4):217–25.
13. Lee MK, Cheng BW, Che CT, Hsieh DP. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol Sci 2000;56(2):424–30.
14. Jang IS, Hwang EH. The Need for Clinical Practice Guidelines in Usage of Mahuang in Weight Loss. Journal of Society of Korean Medicine for Obesity Research 2007;(1):23–29.
15. Department of Health and Human Services, Agency for Healthcare Research and Quality, Shekelle P, Hardy ML, Morton SC, Maglione M, Suttorp M, et al. Ephedra and ephedrine for weight loss and athletic performance enhancement. Clinical efficacy and side effects Available at: URL:
http://www.fda.gov/OHRMS/DOCKETS/98fr/95n-0304-bkg0003-ref-07-01-index.htm
. Accessed June, 2007.
16. Kim HJ, Han CH, Lee EJ, Song YK, Shin BC, Kim YK. A clinical practice guideline for Ma-huang(Ephedra sinica) prescription in obesity. Journal of Society of Korean Medicine for Obesity Research 2007;7(2):27–37.
17. Liu AG, Smith SR, Fujioka K, Greenway FL. The effect of leptin, caffeine/ephedrine, and their combination upon visceral fat mass and weight loss. Obesity 2013;21(10):1991–1996.
18. Kim HJ, Park JM, Kim JA, Ko BP. Effect of Herbal Ephedra sinica and Evodia rutaecarpa on Body Composition and Resting Metabolic Rate: A Randomized, Double-blind Clinical Trial in Korean Premenopausal Women. Journal of Acupuncture and Meridian Studies 2008;1(2):128–138.
19. Hackman RM, Havel PJ, Schwartz JH, Rutledge JC, Watnik MR, Nocite EM, et al. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: A randomized controlled trial. International Journal of Obesity 2006;30(10):1545–1556.
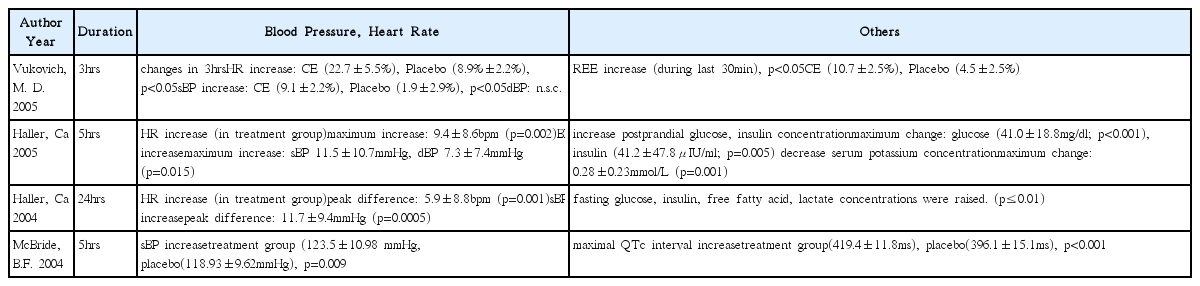
20. Vukovich MD, Schoorman R, Heilman C, Jacob P, Benowitz NL. Caffeine-herbal ephedra combination increases resting energy expenditure, heart rate and blood pressure. Clinical and Experimental Pharmacology and Physiology 2005;32(1–2):47–53.
21. Haller CA, Jacob P, Benowitz NL. Short-term metabolic and hemodynamic effects of ephedra and guarana combinations. Clinical pharmacology and therapeutics 2005;77(6):560–71.
22. McBride BF, Karapanos AK, Krudysz A, Kluger J, Coleman CI, White CM. Electrocardiographic and hemodynamic effects of a multicomponent dietary supplement containing ephedra and caffeine: a randomized controlled trial. Journal of American Medical Association 2004;291(2):216–21.
23. Hioki C, Yoshimoto K, Yoshida T. Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clinical and experimental pharmacology & physiology 2004;31(9):614–9.
24. Haller CA, Jacob P, Benowitz NL. Enhanced stimulant and metabolic effects of combined ephedrine and caffeine. Clinical pharmacology and therapeutics 2004;75(4):259–73.
25. Greenway FL, Jonge L, Blanchard D, Frisard M, Smith SR. Effect of a dietary herbal supplement containing caffeine and ephedra on weight, metabolic rate, and body composition. Obesity Research 2004;12(7):1152–1157.
26. Coffey CS, Steiner D, Baker BA, Allison DB. A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. International Journal of Obesity 2004;28(11):1411–1419.
27. Kalman D, Incledon T, Gaunaurd I, Schwartz H, Krieger D. An acute clinical trial evaluating the cardiovascular effects of an herbal ephedra-caffeine weight loss product in healthy overweight adults. International journal of obesity and related metabolic disorders. journal of the International Association for the Study of Obesity 2002;26:363–1366.
28. Boozer CN, Daly PA, Homel P, Solomon JL, Blanchard D, Nasser JA, et al. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. International journal of obesity and related metabolic disorders. journal of the International Association for the Study of Obesity 2002;26(5):593–604.
29. Zaragoza RM, Lonngi G, Ortiz RA, Huerta DR. Comparison of two formulations of d-norpseudoephedrine and placebo in obese patients treated during six months. Medicina Interna de Mexico 2001;17(6):260–71.
30. Boozer CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. International journal of obesity and related metabolic disorders. journal of the International Association for the Study of Obesity 2001;25(3):316–24.
31. Molnar D, Torok K, Erhardt E, Jeges S. Safety and efficacy of treatment with an ephedrine/ caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents. International journal of obesity and related metabolic disorders. journal of the International Association for the Study of Obesity 2000;24(12):1573–8.
32. Kalman DS, Colker CM, Shi Q, Swain MA. Effects of a weight-loss aid in healthy overweight adults: Double-blind, placebo-controlled clinical trial. Current Therapeutic Research - Clinical and Experimental 2000;61(4):199–205.
33. Jang IS, Yang CS, Hwang EH. The need for clinical practice guidelines in usage of Mahuang in weight loss. Journal of Society of Korean Medicine for Obesity Research 2007;7(1):23–29.