References
1. The co-textbook publishing committee of Korean Medicine College. Formula Science Seoul: Younglimsa; 2009.
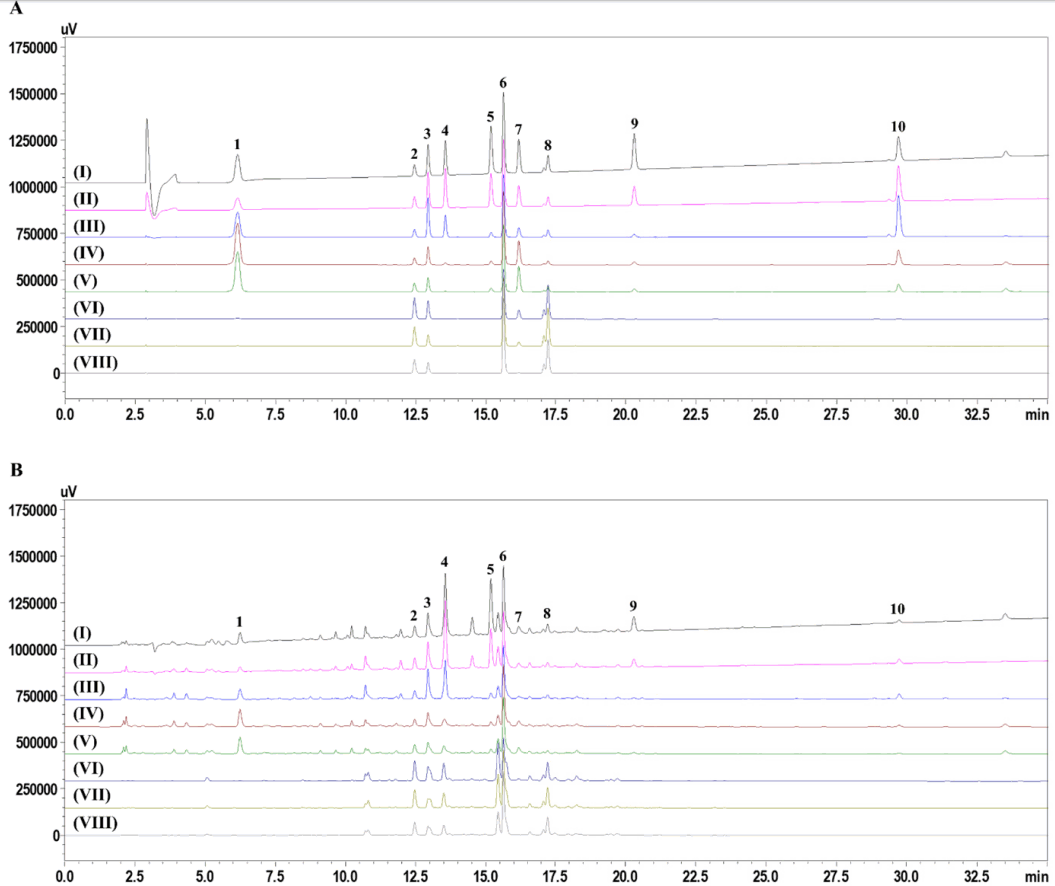
2. Seo CS, Shin HK, Kim JH, Shin KS. Changes of Principal Components and Microbial Population in Pyungwi-san Decoction According to the Preservation Temperature and Period. J Korean Oriental Med 2011;32(5):41–9.
3. Ha H, Shin IS, Lim HS, Jeon WY, Kim JH, Seo CS, Shin HK. Changes in Anti-inflammatory Effect of Pyungwi-san Decoction According to the Preservation Temperature and Period. Formula Science 2012;20(2):29–35.
4. Seo CS, Kim JH, Lim SH, Shin HK. Establishment of Shelf-Life of Ssanghwa-tang by Long-term Storage Test. Kor J Pharmacogn 2012;43(3):257–64.
5. Seo CS, Kim JH, Kim SS, Lim SH, Shin HK. Evaluation of Shelf-life of Bojungikgi-tang by Long-term Storage Test. Kor J Pharmacogn 2013;44(2):200–8.
6. Jin SE, Kin OS, Shin HK, Jeong SJ. Comparative Study on Biological Activities of Gwakhyangjeonggi-san Decoction According to the Preservation Periods. J Korean Med 2014;35(3):60–9.
7. Jin SE, Kim OS, Seo CS, Shin HK, Jeong SJ. Comparative study on stability and efficacy of Banhasasim-tang decoction depending on the preservation temperature and periods. J Korean Med 2016;37(1):21–33.
8. Yoo SR, Ha H, Lee NR, Shin HK, Seo CS. A Study on Change of Marker Compounds and Biological Activity in Chungsimyeonja-eum Decoction Depending on A Storage Temperature and Periods. Kor J Herbol 2017;32(4):25–32.
9. Park IH, Kim YH, Choi SH, Yu SN, Kim SH, Ahn SC, Cho SI, Lee I. Effect of Preservation Conditions on the Stability of Samul-tang Decoctions. J Life Sci 2015;25(10):1124–31.
10. Do HJ, Shim YS, Lee JH, Ahn YJ, Ha IH, Lee YJ, et al. Stability of Danggwisu-san (Dangguixu-san) Water Extract, a Herbal Medicine, Under Various Storage Conditions. Journal of Oriental Rehabilitation Medicine 2016;26(4):1–8.
11. Jin SM. Taepyunghyeminhwajekukbang Inminweeseng Publications; 1985. p. 308.
12. Sim TK, Jung IC, Lee SR. The Effect of Gami soyo-san(Jiaweixiaoyaosan) on Serotonin Metabolism. Journal of Oriental Neuropsychiatry 2011;22(1):37–51.
13. Lee SL, Lee IS, Soh KS. Effects of Gamisoyosan(Jiaweixiaoyaosan 加味逍遙散) on Type II Collagen-Induced Arthritis. J Oriental Rehab Med 2001;12(4):167–76.
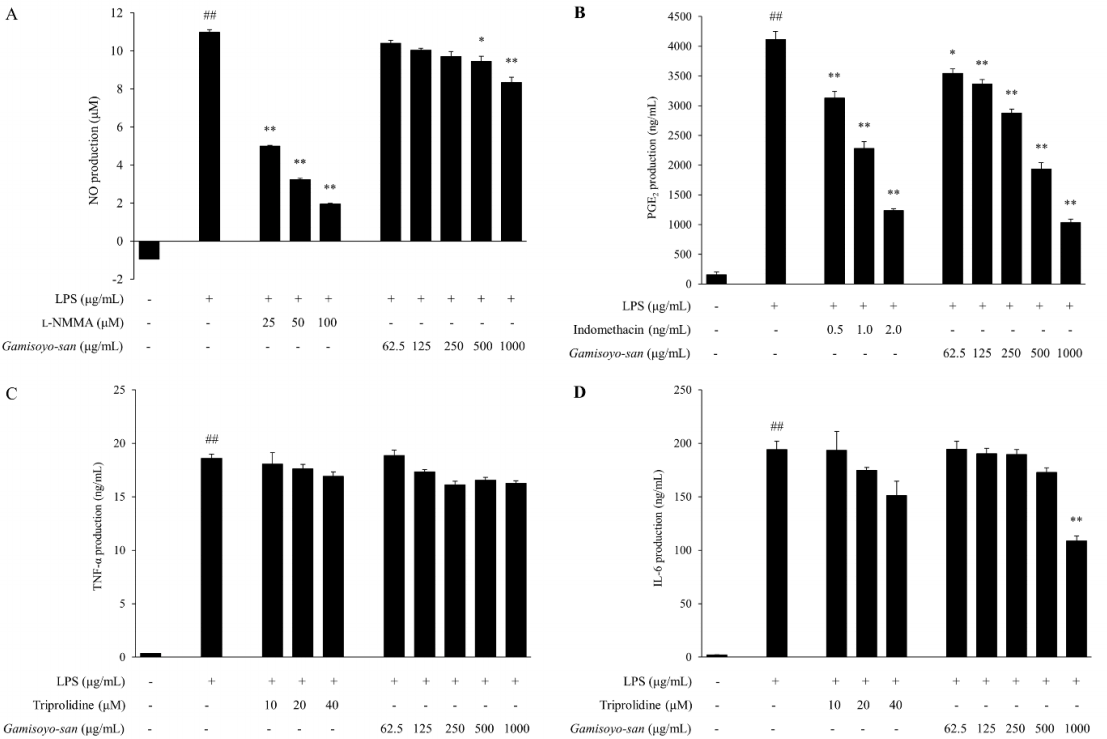
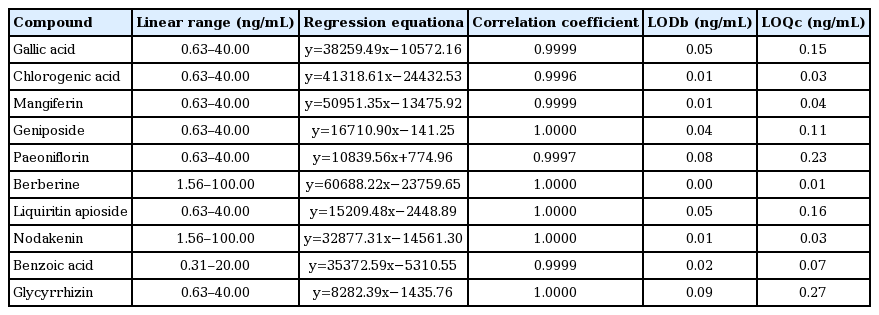
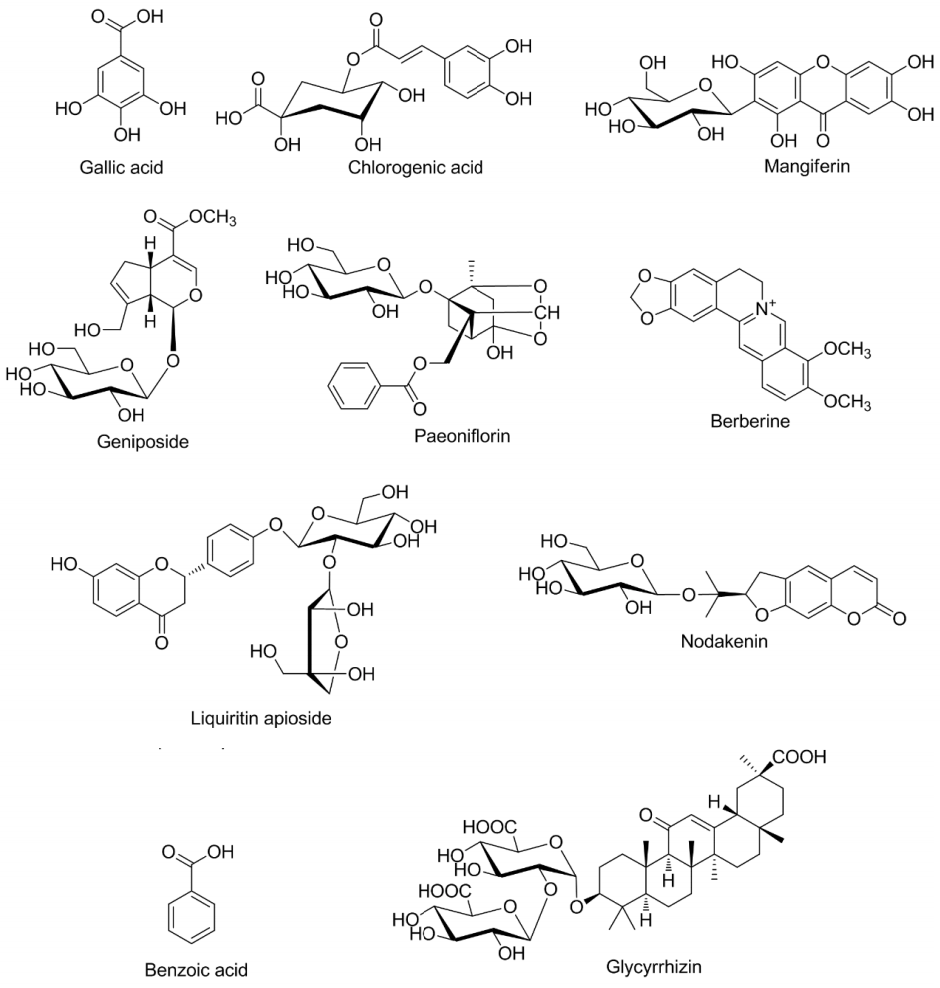
14. Jin SE, Kim OS, Yoo SR, Seo CS, Kim Y, Shin HK, et al. Anti-inflammatory effect and action mechanisms of traditional herbal formula Gamisoyo-san in RAW 264.7 macrophages. BMC Complement Altern Med 2016;16:219. 10.1186/s12906-016-1197-7.
15. Choi HM, Kim SJ, Kim IS, Lee JB, Kim JB, Moon SO, et al. Evaluation on Anti-oxidant Activity and Anti-inflammatory Effects for the New Formulation of Gamisoyosan. Kor J Herbol 2016;31(6):1–9.
16. Willoughby DA. Human arthritis applied to animal models. Towards a better therapy. Ann Rheum Dis 1975;34:471–8.
17. Lee ES, Ju HK, Moon TC, Lee E, Jahng Y, Lee SH, et al. Inhibition of nitric oxide and tumor necrosis factor α (TNF-α) production by propenone compound through blockade of nuclear factor (NF)-κB activation in cultured murine macrophages. Biol Pharm Bull 2004;27:617–20.
18. Wink DA. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 1998;25(4–5):434–56.
19. Ryu JH, Ahn H, Kim JY, Kim YK. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophages. Phytother Res 2003;17(5):485–9.
20. Funk CD, Fitzgerald GA. Human platelet, erythroleukemia cell prostaglandin G, H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J 1991;5:2304–12.
21. Bishop-Bailey D, Calatayud S, Warner TD, Hla T, Michell A. Prostaglandins and the regulation of tumor growth. J Environ Pathol Tox Oncol 2002;21:93–101.
22. Feldmann M. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996;14:397–440.
23. Mann JR, Backlund MG, DuBois RN. Mechanism of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol 2005;2:202–10.
24. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Phamacol 2003;43:469–87.
25. Chiu CS, Deng JS, Chang HY, Chen YC, Lee MM, Hou WC, et al. Antioxidant and anti-inflammatory properties of Taiwanese yam (Dioscorea japonica Thunb. var. pseudojaponica (Hayata) Yamam.) and its reference compounds. Food Chem 2013;141:1087–96.
26. Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res 2014;63:81–90.
27. Garrido G, Delgado R, Lemus Y, Rodríguez J, García D, Núñez-Sellés AJ. Protection against septic shock and suppression of tumor necrosis factor alpha and nitric oxide production on macrophages and microglia by a standard aqueous extract of Mangifera indica L. (VIMANG). Role of mangiferin isolated from the extract. Pharmacol Res 2004;50:165–72.
28. Shi Q, Cao J, Fang L, Zhao H, Liu Z, Ran J, et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kB, MAPK and AP-1 signaling pathways in macrophages. Int Immunopharmacol 2014;20:298–306.
29. Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol 2014;52(9):1189–95.
30. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab 2009;296:E955–64.
31. Guan Y, Li FF, Hong L, Yan XF, Tan GL, He JS, et al. Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury. Fundam Clin Pharmacol 2012;26(4):473–83.
32. Rim HK, Cho W, Sung SH, Lee KT. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-kB pathways and protects mice from lethal endotoxin shock. J Pharmacol Exp Ther 2012;342:654–64.
33. Fu Y, Zhou E, Wei Z, Liang D, Wang W, Wang T, et al. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J 2014;281:2543–57.