References
1. National Professors of Bikye Internal Medicine. Bikye Internal Medicine Seoul: Kunja Publisher; 2009. 96p. 314–318.
2. Cho YS, Choi MG, Jeong JJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Asan-si, Korea. Am J Gastroenterol 2005;100:747–753.
3. Lee D, Lee KJ, Kim KM, Lim SK. Prevalence of asymptomatic erosive esophagitis and factors associated with symptom presentation of erosive esophagitis. Scand J Gastroenterol 2013;48:906–912.
4. Kim JH, Kim JB, Kim SY, Kim SE, Kim YS, Sung HY, et al. Current Issues on Gastroesophageal Reflux Disease. Korean J Gastroenterol 2014;64:127–128.
5. Jung HK, Hong SJ, Jo YJ, Jeon SW, Cho YK, Lee KJ, et al. Updated guidelines 2012 for gastroenterol reflux disease. Korean J Gastroenterol 2012;60(4):195–218.
6. National Professors of Herbology. Herbology Seoul: Youngleem Publisher; 1998. p. 475. p. 513. p. 632.
7. Anderson T, Rohss K, Bredberg E, Hassan-Alim M. Pharmacokinetics and pharmacodynamics of esomeprasole, the S-isomer of omeprasole. Aliment Pharmacol Ther 2001;15:1563–1569.
8. Nakamura k, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodiumpolyacrylate(PANa) on acute esophagitis by gastric juice in rats. Japan J Pharmacol 1982;32:445–456.
9. Sin GT. Steps to Internal Medicine 6 Gastrodigestive Disease Seoul: Jungdam Publisher; 2002. p. 54–55.
10. Horn J. The proton-pump inhibitors: similarities and differences. Clinther 2000;22:266–280.
11. Kin HJ, Leem SY, Gwak MA, Kim DJ, Byeon JS. Effects of Yijintang-gamibang on Reflux Esophagitis Induced by Pylorus and Forestomach Ligition in Rat. J of Internal Korean Medicine 2010;31(1):128–141.
12. Heo JI. Protective Effects of Yijin-tang-gamib-molyeo Aqueous Extracts on Reflux Esophagitis. PhD Dissertation Daegu: Daegu Haany University; 2011.
13. Dent J. Pharmacology of esomeprazole and comparisons with omeprazole. Aliment Pharmacol Ther 2003;17(suppl 1):5–9.
15. Fass R, Dickman R. Non erosive reflux disease. GI Motility online 2006;10.1038/gimo42.
16. Fauci. Harrison’s Internal Medicine Seoul: MIP Publisher; 2010. p. 2234.
17. Park K, Gwak MA, Kim DJ, Byeon JS. Protective Effects of Yijin-tang-gamibang Aqueous Extracts on Reflux Esophagitis Mediated by Antioxidant Defense Systems. Korean J of Oriental Physiology and Pathology 2010;24(3):416–425.
18. Jang MW, Leem SW. Experimental Study for Effect of Banhasasim-tang on Mice with Reflux Esophagitis. J of Internal Korean Medicine 2013;34(4):362–374.
19. Kim MY, Sin YO, Lee JY, Lee AR, Sin SH, Kwon OJ, et al. Improving Effect of a Combined Extract of Rhei Rhizoma and Glycyrrhizae Rhizoma through Anti-oxidative Stress in Reflux Esophagitis rats. Korea J of Herbology 2015;30(4):37–44.
20. Kim DJ, No SS. Effect on Acute reflux Esophagitis by Evodiae Fructus Aquous Extract. Korea J of Herbology 2012;27(1):51–58.
21. Lee YJ, Park JH, No SS. Effects on Rats with Reflux Esophagitis Treated with Lonicerae Flos Extract. Korean J of Oriental Physiology and Pathology 2010;24(6):970–975.
22. Chinese Medicine Dictionary Publish Members. Chinese Medicine Dictionary Seoul: Jungdam Publisher; 1997. p. 1317–1320.
p. 3123–3124.
p. 4722–4726.
23. Lee NK, Yun HI, Park SC, Park JI, Jo MH. Effects of heat-treated alumen, halloysitum rubrum and os sepiae in experimentally induced stomach ulcer in rats. J of Applied Pharmacology 1997;5:246–252.
24. Kim TG, Lee YH, Choi YS, Byeon JS, Park SD. The Effects of ShogunJungtang-ga-younggol. morea on Gastric Ulcer. J of Internal Korean Medicine 2001;22(1):13–20.
25. Patk SM, Jo YG, Kee JR, Lee YC, Kim HJ, Kwon YK, et al. Inhibitory Effect of Oyster Conchioloin on Pro-inflammatory Mediator in Lipopolysaccharide;Activated Raw 264.7 Cells. Korean J of Oriental Physiology and Pathology 2008;22(4):878–883.
26. Lee YT, Choi BT, Choi Yh, Kang KH. Development of Health Assistances for Anti Stress used with Ostreae Concha. Korean J of Oriental Physiology and Pathology 2006;20(6):1604–1611.
27. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287(1):G7–17.
28. Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox activated forms. Science 1992;258:1898–1902.
29. De May JG, Vanhoutte PM. Rolo of the intima in cholinergic and purinergic relaxation of isolated canin femoral arteries. Journal of Physiology 1981;316:347–355.
30. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–376.
31. Brennan FM, Maini RN, Feldmann M. Cytokine expression in chronic inflammatory disease. Br Med Bull 1995;51(2):368–84.
32. Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem 18. 269(7):4705–8.
1994;
33. Barnes PJ, Karin MN. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. Engl J Med 10. 336(15):1066–71.
1997;
34. Baeuerle PA, Baltimore D. NF-κB - Ten years after. Cell 1996;87:13–20.
35. Seo WG, Pae HO, Oh GS, Chai KY, Yun YG, Kwon TO, et al. Inhibitory effect of ethyl acetate fraction from Cudrania tricuspidata on the expression of nitric oxide synthase gene in RAW 264.7 macrophages stimulated with interferon-and lipopolysaccharide. Gen Pharmacol 2000;35:21–28.
36. Seo WG, Pae HO, Oh GS, Chai KY, Kwon TO, Yun YG, et al. Inhibitory effects of methanol extracts of Cyrerus rotundus rhizomes on nitric oxide and superoxide production by murine macrophages cell line, RAW 264.7 cells. J Ethnopharmacol 2001;76:59–64.
37. Lee BG, Kim SH, Zee OP, Lee KR, Lee HY, Han JW, et al. Suppression of inducible nitric oxide synthase expression in RAW 264.7 macrophagesby two-carboline alkaloids extractes from Melia azedarach. Eur J Pharmacol 2000;406:301–309.
38. Lee DA, Lee JR, Kim YW, Kwon YK, Byun SH, Sin SW, et al. Inhibition of Lipopolysaccharide-Inducible Nitric Oxide Synthase, TNF-α, IL-1β and COX-2 Expression by Flower and Whole Plant of Lonicera japonica. Korean J of Oriental Physiology and Pathology 2005;19(2):481–489.
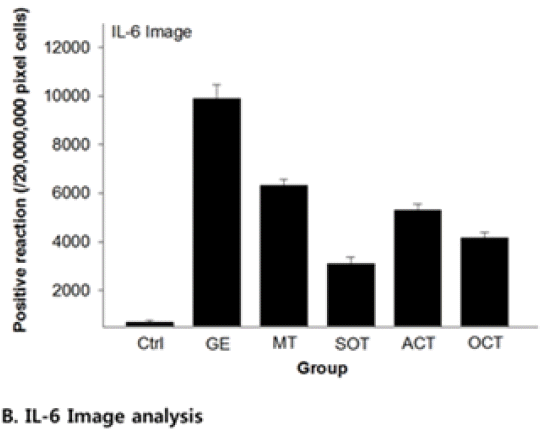
39. Byeon SH, Yang JH, Kim SC. Inhibitory effect of Scrophulariae Radix extract on TNF-a, IL-1β, IL-6 and Nitric Oxide production in Lipopolysaccharide - activated Raw 264.7 cells. Korea J of Herbology 2005;20(2):7–16.
40. Racke K, Riemann A, Schworer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cell. Behav Brain Res 1996;73:83–7.
41. Morrow JD, Roberts LJ. Lipid-derived autocoides, eicosanoids and platelets-activating factor. In : Hardman JG, Limberd LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics USA: McGraw Hill and Co; 2001. p. 673.
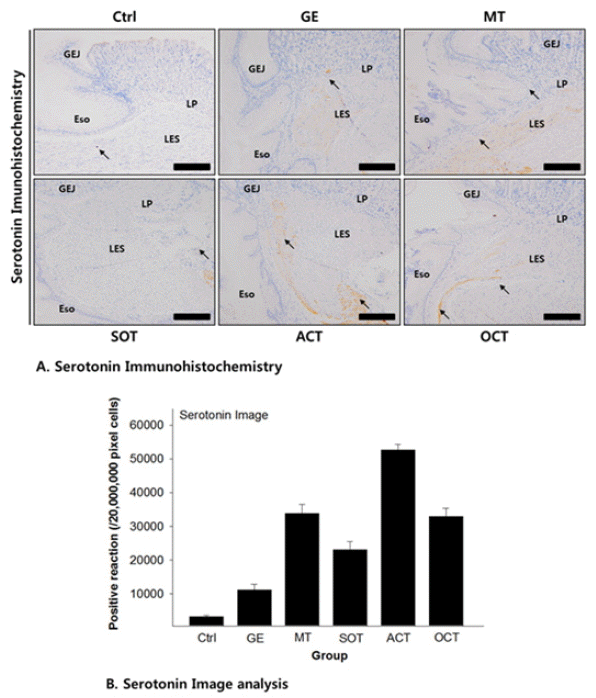
42. Saegusa Y, Takeda H, Muto S, Oridate N, Nakagawa K, Sadakane C, et al. Decreased motility of the lower esophageal sphincter in a rat model of gastroesophageal reflux disease may be mediated by reductions of serotonin and acetylcholine signaling. Biol Pharm Bull 2011;34(5):704–11.
43. Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphlogical feature of acid reflux – damaged human esophageal epithelium. Gastroenterology 1996;111(5):1200–5.
44. De Hertogh G, Ectors N, Van Eyken P, Geboes K. Review article: the nature of oesophageal injury in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2006;24(Suppl2):17–26.
45. Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs 2005;65(16):2253–86.
46. Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox activated forms. Science 1992;258:1898–1902.
47. De May JG, Vanhoutte PM. Rolo of the intima in cholinergic and purinergic relaxation of isolated canin femoral arteries. Journal of Physiology 1981;316:347–355.
48. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–376.
49. Han GJ, Leem JT, Kim JS, Lee JH. Analysis of the Existing Guidelines and Clinical Trials for the Development of the Guidelines of Clinical Trials with Herbal Medicinal Products for Gastroesophageal Reflux Disease(GERD). J of Internal Korean Medicine 2016;37(1):90–108.