A Comparative Study on the Effects of Pinellia ternata, Zingiber officinale and Sobanhatang on Reflux Esophagitis
Article information
Abstract
Objectives
This study was carried out to observe and compare the effects of Pinellia ternata, Zingiber officinale and Sobanhatang on the reflux esophagitis induced by gastric fundus and pylorus ligation in mice with esomeprazole.
Methods
Antioxidant effects were measured by DPPH radical scavenging activity at four different concentration of 0.125, 0.25, 0.5 and 1.0mg/102μℓ. Zingiber officinale water extract(ZE), Pinellia ternata water extract(PE) and Sobanhatang water extract(SBE) and esomeprazole were treated orally for 14 days before gatric fundus and pylorus ligation. In the histochemistry, changes in suface mucous cells, muscle tissue and connective tissue in gastro esophageal junction(GEJ) and mast cell on the esophageal mucosa were observed. The change of Hemo oxygenase(HO)-1, ghrelin, gastrin and substance P in gastric body tissue were measured by immunohistochemistry.
Results
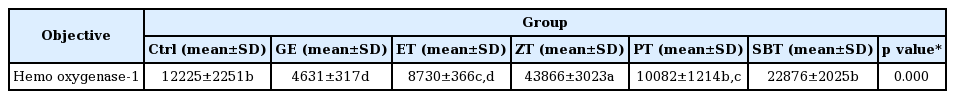
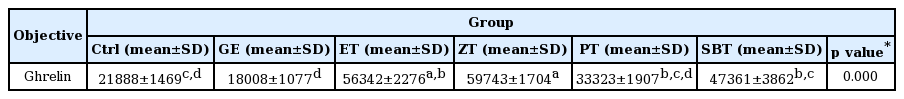
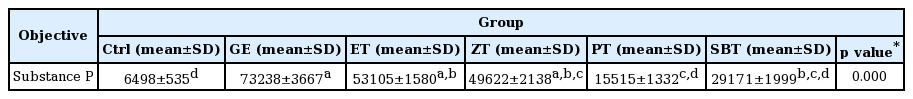
DPPH radical scavenging activity exhibited concentration dependently increases in ZE, PE, SBE. ZE was significantly higher at all concentrations than PE. The gastric surface mucous cells were more in the treated group than in the reflux esophagitis elicited group(GE) in the order of PE, SBE, ZE and esomeprazole treateded group(PT, SBT, ZT, ET). Lower esophageal sphincter muscle damage and intercellular space in the GEJ were less in the treated group than GE. In the esophageal mucosa, the mast cell distribution and the migration of inflammatory cells were lower in the treateded troup than GE in order to ZT, SBT, PT and ET. The antioxidative enzyme, HO-1 was more in the order of ZT, SBT, control group, PT, ET than in GE. ZT was significantly higher than the other groups and SBT was significantly higher than ET. Ghrelin was found to be higher in ZT, ET, SBT and PT than in GE, and ZT was significantly higher than all other groups except ET. Gastrin showed the highest positivity in GE, and was lower in the order of ET, ZT, SBT, PT, and control group. Substance P was the highest in GE, and was lower in the order of ET, ZT, SBT, PT and control group, and PT were significantly lower than ET.
Conclusion
ZT, PT and SBT showed superior antioxidative, anti-inflammatory and mucosal protective effects on mouse reflux esophagitis as compared with ET. In particular, ZE was more effective in antioxidant and gastric motility enhancement, while PE was more effective in mucosal protection and anti-inflammatory effects. Sobanhatang is expected to be effective treatment because it has advantages of both drugs and reduces toxicity.
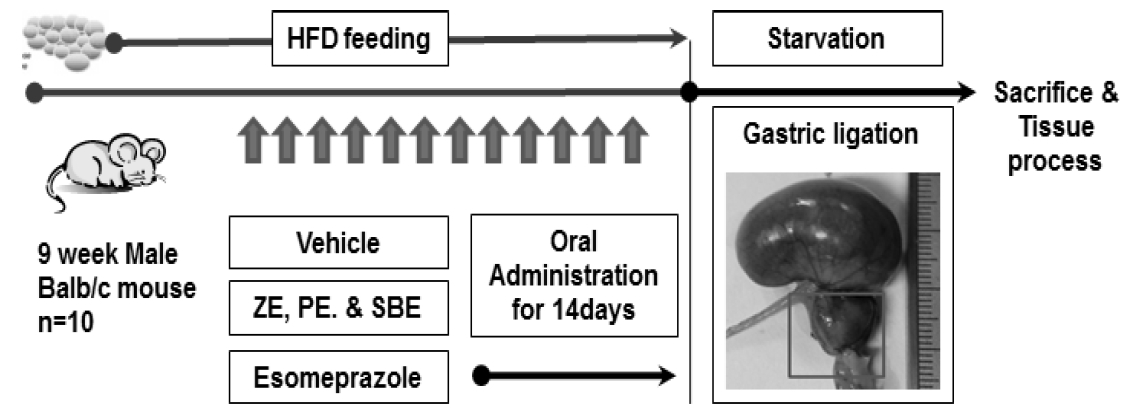
Experimental design and reflux esophagitis animal model
HFD, high fat diet; ZE, Zingiber officinale water extract; PE, Pinellia ternata water extract; SBE, Sobanhatang water extract.
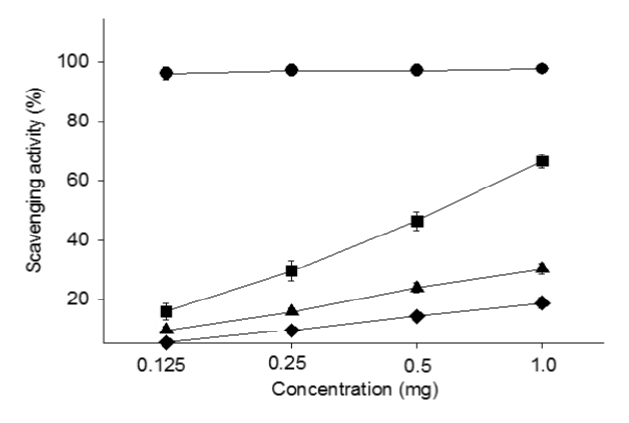
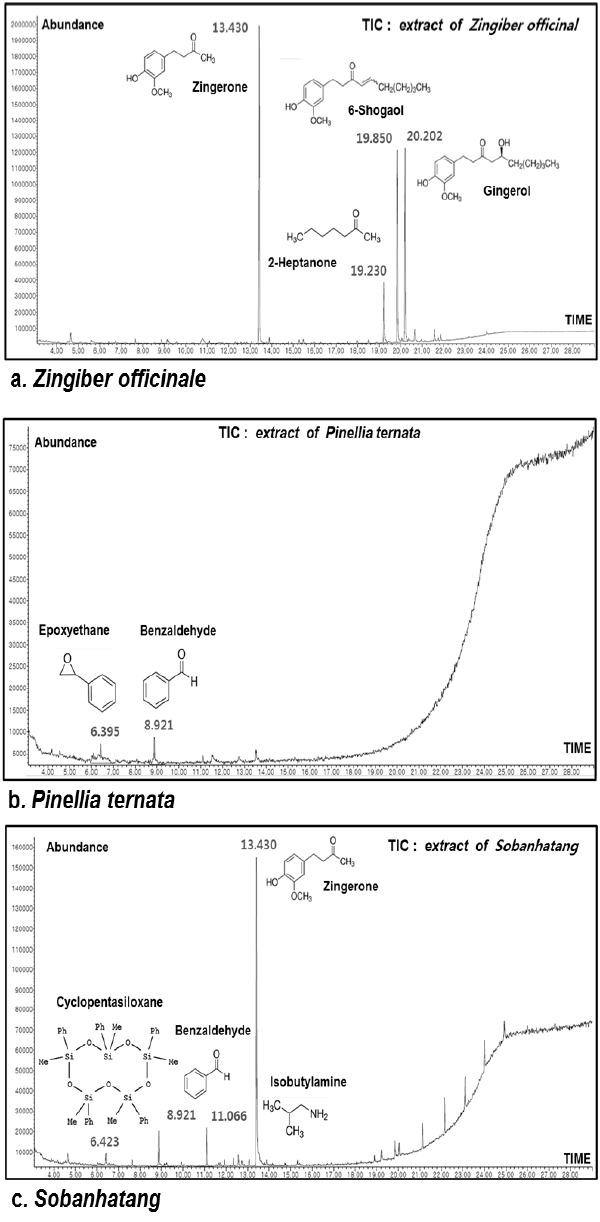
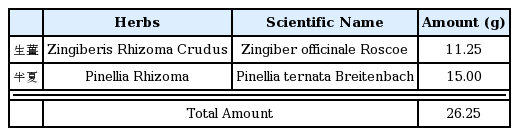
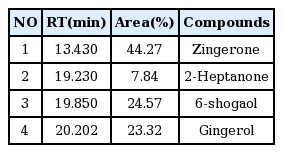
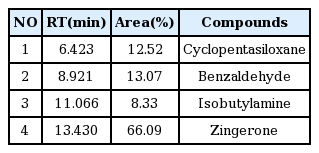
The free radical scavenging activity of water extracts of Pinellia ternata, Zingiber officinale and Sobanhatang
The anti-oxidant ability of ZE and SBE is dose-dependantly increased and the estimation of SBE is lower compared with ZE. ♦, Pinellia ternata water extract(PE); ■, Zingiber officinale water extract(ZE); ▲, Sobanhatang water extract(SBE); ●, Ascorbic acid as compared anti-oxidant.
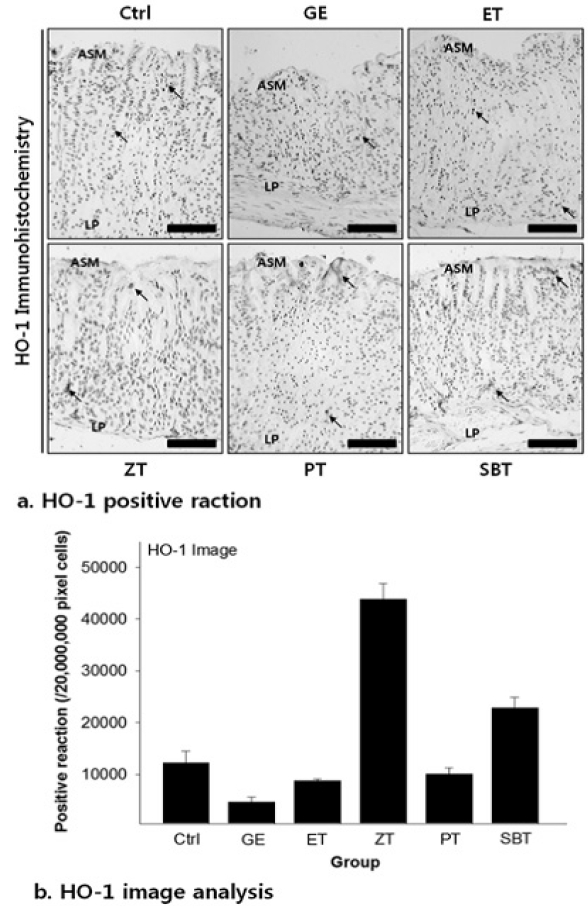
The hemo oxygenase-1 positive reaction in gastric mucosa with reflux esophagitis
Ctrl, no reflux esophagitis elicited group; GE, reflux esophagitis elicited group; ET, esomeprazole treated group before reflux esophagitis elicitation; ZT, Zingiber officinale water extract treated group before reflux esophagitis elicitation; PT, Pinellia ternata water extract treated group before reflux esophagitis elicitation; SBT, Sobanhatang water extract treated group before reflux esophagitis elicitation; Bar size, 100μm; arrow, HO-1 positive reaction; ASM, apical surface of mucosa, LP, lamina propria.
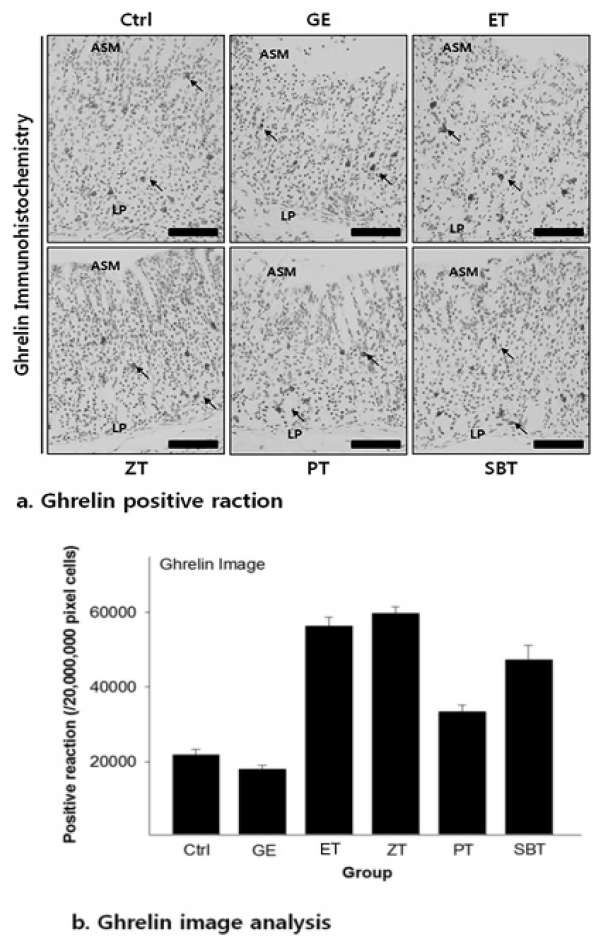
The ghrelin positive reaction in gastric mucosa with reflux esophagitis
Bar size, 100μm; arrow, ghrelin positive reaction; ASM, apical surface of mucosa, LP, lamina propria.
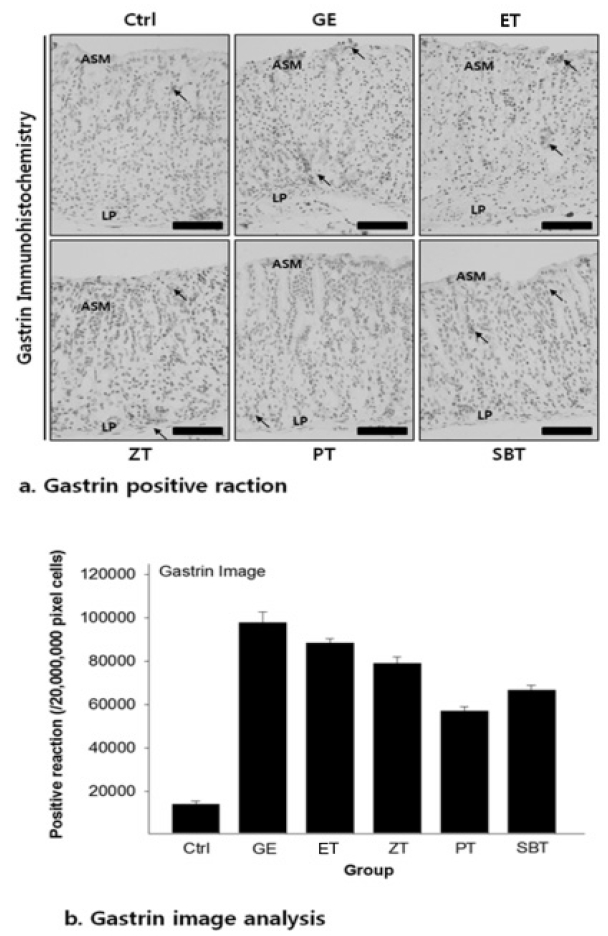
The gastrin positive reaction in gastric mucosa with reflux esophagitis
Bar size, 100μm; arrow, gastrin positive reaction; ASM, apical surface of mucosa, LP, lamina propria.
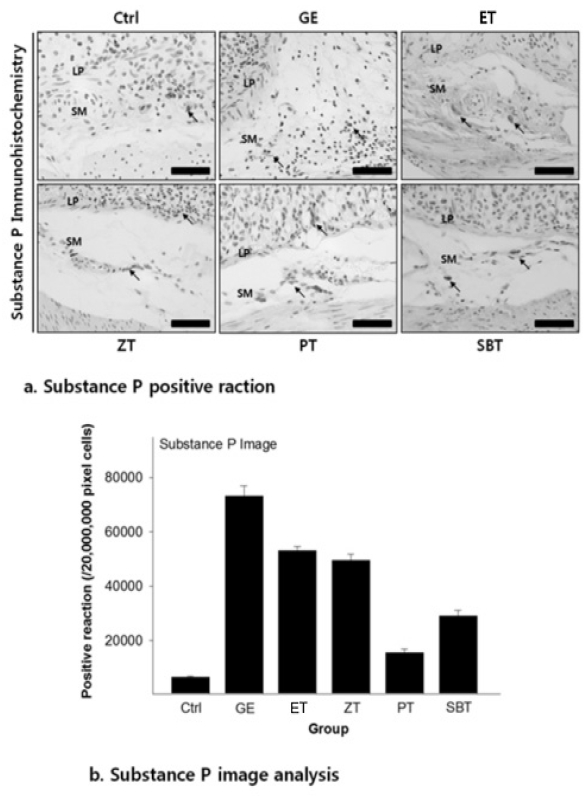
The substance P positive reaction in gastric mucosa with reflux esophagitis
Bar size, 100μm; arrow, gastrin positive reaction; ASM, apical surface of mucosa, LP, lamina propria
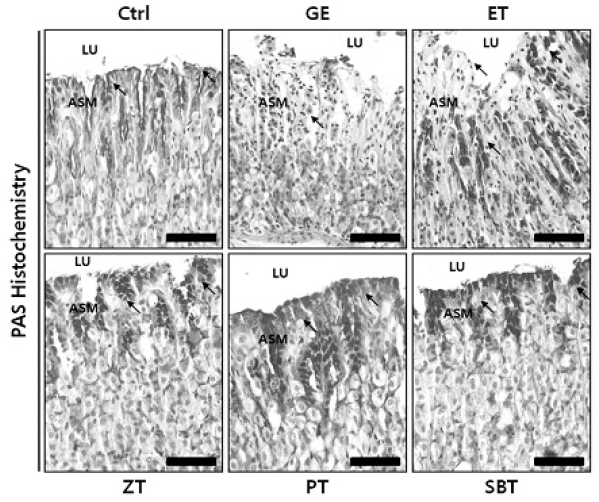
The protective effect of damaged mucosal barrier by Pinellia ternata water extract, Sobanhatang water extract, Zingiber officinale water extract and esomeprazole
The PAS positive reaction (arrow) in mice pre-treateded with PE, SBE, ZE and esomeprazole noticeably maintain (PAS stain; Bar size, 50μm).
ASM, apical surface of mucosa; LU, lumen.

The mitigative effect of muscular damage & mucosal inflammation by Pinellia ternata water extract, Sobanhatang water extract, Zingiber officinale water extract and esomeprazole
A photo Bar size, 200μm; B photo Bar size, 50μm, GEJ, gastroesophageal junction; LES, lower esophageal sphincter; ES, esophagus; SM, submucosa; LU, lumen.

DPPH Radical Scavenging Activity of Water Extracts of Pinellia ternata, Zingiber officinale and Sobanhatang Scavenging activity (%)
Notes
이 논문은 2017년도 상지대학교 대학원 박사학위 논문임