References
1. Verroken M. Drug use and abuse in sport. Baillieres Best Pract Res Clin Endocrinal Metab 2000;14:1–23.
2. Kim JH. Sports-moral and ethical aspects Seoul: Rainbowbooks; 2015. p. 97–9.
3. Lee HS. Sports sociology Seoul: Sungshin press. 2004p. 301.
4. Kim JR. A counter-strategy of Korea for anti-doping policy in world sports [dissertation] Seoul: Hansung University; 2002.
5. Judkins C, Prock P. Supplements and inadvertent doping - how big is the risk to athletes. Med Sport Sci 2012;59:143–52.
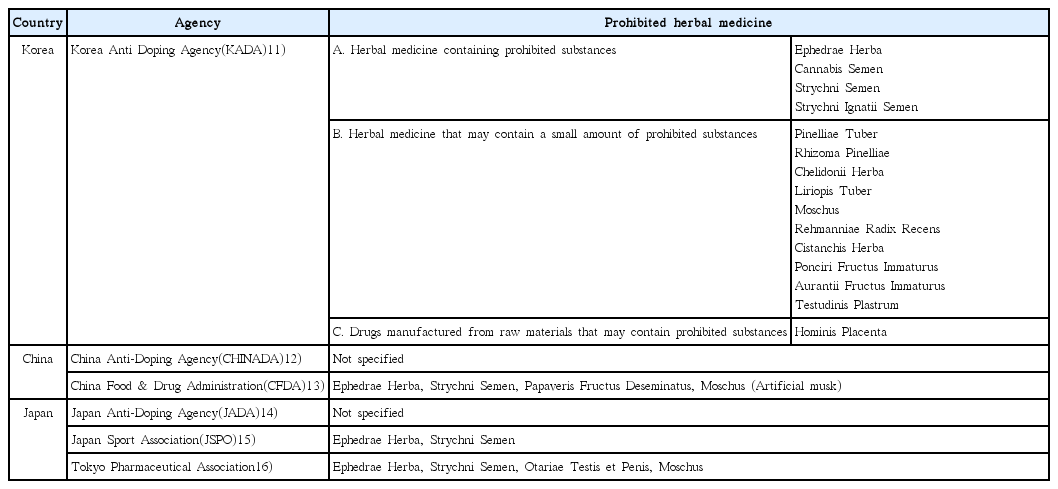
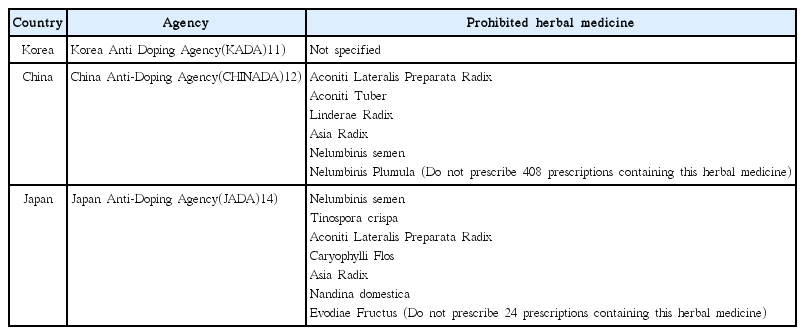
6. Kim JK, Chun YS, Kang SK, Cho HC. The use of herbal/traditional products supplementation and doping tests in elite athletes. Korean Journal of Sport Science 2009;20(4):734–42.
7. Yun SJ, Je JJ, Lee H, Kim YJ, Lee HS. Anti-dopping of herbal medicine in 2015. J Sports Korean Med 2015;15(1):1–9.
8. Kim JK, Kang SK, Jung HS, Chun YS, Trilk J, Jung SH. Dietary supplementation patterns of Korean olympic athletes participating in the Beijing 2008 summer olympic games. Int J Sport Nutr Exerc Metab 2011;Apr. 21(2):166–74.
9. Yun SJ. Anti-doping of herbal medicine in 2018. The Korean Medicine Times 2018;Jul. 2. 2171:23. (col. 1).
10. World anti-doping agency(WADA) [Internet] Montreal(Quebec): WADA; 2018. c2018. [cited 2018 Oct 3]. Available from:
https://www.wada-ama.org/
.
11. Korea anti doping agent [Internet] Seoul: KOREA ANTI-DOPING AGENCY; c2014. [cited 2018 Oct 3]. Available from:
https://www.kada-ad.or.kr/
.
12. China anti-doping agency [Internet] Beijing: CHINADA; c2015–2016. [cited 2018 Oct 3]. Available from:
http://www.chinada.cn/
.
19. Andraws R, Chawla P, Brown DL. Cardiovascular effects of ephedra alkaloids: A comprehensive review. Prog Cardiovasc Dis 2005;Jan–Feb. 47(4):217–25.
20. Herbal Pharmacology textbook compilation committee. Herbal pharmacology 4th edth ed. Seoul: Shinilbooks; 2015. p. 202–7.
p. 260–5.
p. 348–64.
p. 365–9.
p. 733–5.
p. 799–803.
p. 877–80.
p. 934–7.
21. Kim HC. Herbal medicine pharmacology Seoul: Jipmoon; 2001. p. 63–6.
p. 180.
22. Rice J, Proctor K, Lopardo L, Evans S, Kasprzyk-Hordern B. Stereochemistry of ephedrine and its environmental significance: Exposure and effects directed approach. J Hazard Mater 2018;Apr. 15. 348:39–46.
23. Soni MG, Carabin IG, Griffiths JC, Burdock GA. Safety of ephedra: Lessons learned. Toxicol Lett 2004;Apr. 15. 150(1):97–110.
24. Maglione M, Miotto K, Iguchi M, Jungvig L, Morton SC, Shekelle PG. Psychiatric effects of ephedra use: An analysis of food and drug administration reports of adverse events. Am J Psychiatry 2005;Jan. 162(1):189–91.
25. Song YK, Lim HH. Clinical application of ma huang in the obesity treatment. Journal of Society of Korean Medicine for Obesity Research 2007;7(1):1–7.
26. Astrup A, Toubro S. Thermogenic, metabolic, and cardiovascular responses to ephedrine and caffeine in man. Int J Obes Relat Metab Disord 1993;Feb. 17(Suppl 1):S41–3.
27. Wilkinson GR, Beckett AH. Absorption, metabolism, and excretion of the ephedrines in man. II. Pharmacokinetics. J Pharm Sci 1968;Nov. 57(11):1933–8.
28. Haller CA, Jacob P 3rd, Benowitz NL. Pharmacology of ephedra alkaloids and caffeine after single-dose dietary supplement use. Clin Pharmacol Ther 2002;Jun. 71(6):421–32.
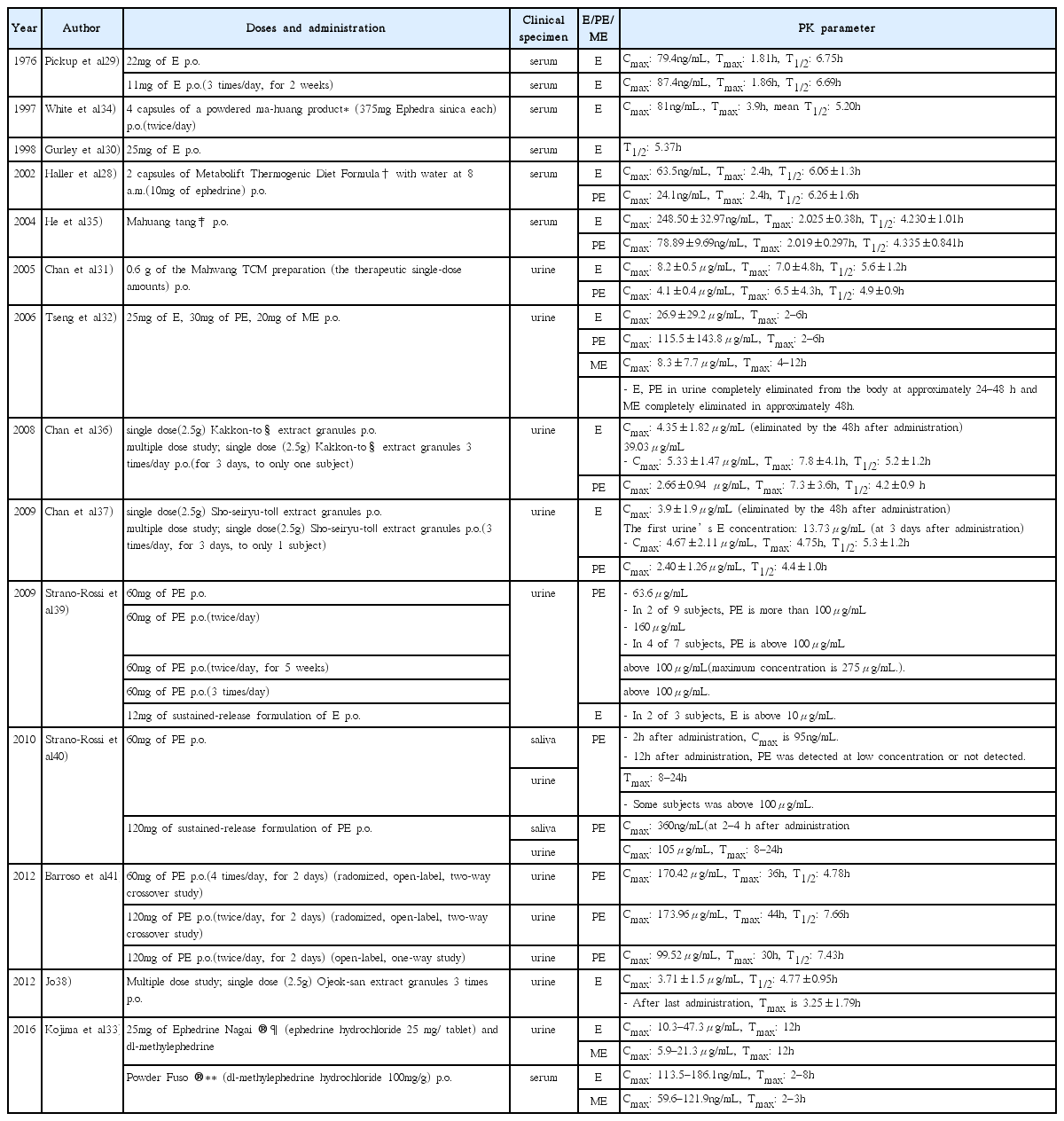
29. Pickup ME, May CS, Ssendagire R, Paterson JW. The pharmacokinetics of ephedrine after oral dosage in asthmatics receiving acute and chronic treatment. Br J Clin Pharmacol 1976;Feb. 3(1):123–34.
30. Gurley BJ, Gardner SF, White LM, Wang PL. Ephedrine pharmacokinetics after the ingestion of nutritional supplements containing Ephedra sinica (ma huang)
. Ther Drug Monit 1998;Aug. 20(4):439–45.
31. Chan KH, Pan RN, Hsu MC. Simultaneous quantification of six ephedrines in a Mahwang preparation and in urine by high–performance liquid chromatography. Biomed Chromatogr 2005;Jun. 19(5):337–42.
32. Tseng YL, Shieh MH, Kuo FH. Metabolites of ephedrines in human urine after administration of a single therapeutic dose. Forensic Sci Int 2006;Mar. 10. 157(2–3):149–55.
33. Kojima A, Nishitani Y, Sato M, Kageyama S, Dohi M, Okano M. Comparison of urine analysis and dried blood spot analysis for the detection of ephedrine and methylephedrine in doping control. Drug Test Anal 2016;Feb. 8(2):189–98.
34. White LM, Gardner SF, Gurley BJ, Marx MA, Wang PL, Estes M. Pharmacokinetics and cardiovascular effects of ma–huang (Ephedra sinica) in normotensive adults. J Clin Pharmacol 1997;Feb. 37(2):116–22.
35. He F, Luo J, Chen F, Yu L. Human pharmacokinetics of ephedrine and pseudoephedrine in Mahuang Tang by GC-MS. Traditional Chinese Drug Research and Clinical Pharmacology 2004;Sep. 15(5):336–8.
:347.
36. Chan KH, Pan RN, Hsu MC, Hsu KF. Urinary elimination of ephedrines following administration of the traditional Chinese medicine preparation Kakkon-to. J Anal Toxicol 2008;Nov–Dec. 32(9):763–7.
37. Chan KH, Hsu MC, Chen FA, Hsu KF. Elimination of ephedrines in urine following administration of a Sho-seiryu-to preparation. J Anal Toxicol 2009;Apr. 33(3):162–6.
38. Jo YK. The study on anti-doping stability of ojeok-san by measuring ephedrine concentration of urine after taking ojeok-san [dissertation] Seoul: KyungHee University; 2012.
39. Strano-Rossi S, Leone D, de la Torre X, Botre F. The relevance of the urinary concentration of ephedrines in anti-doping analysis: Determination of pseudoephedrine, cathine, and ephedrine after administration of over-the-counter medicaments. Ther Drug Monit 2009;Aug. 31(4):520–6.
40. Strano-Rossi S, Leone D, de la Torre X, Botre F. Analysis of stimulants in oral fluid and urine by gas chromatography-mass spectrometry II: Pseudophedrine. J Anal Toxicol 2010;May. 34(4):210–5.
41. Barroso O, Goudreault D, Carbo Banus ML, Ayotte C, Mazzoni I, Boghosian T, et al. Determination of urinary concentrations of pseudoephedrine and cathine after therapeutic administration of pseudoephedrine-containing medications to healthy subjects: Implications for doping control analysis of these stimulants banned in sport. Drug Test Anal 2012;May. 4(5):320–9.
42. Lee MK, Cheng BW, Che CT, Hsieh DP. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol Sci 2000;Aug. 56(2):424–30.
43. Lee NR, Ha HK, Shin HK, Seo CS. Quantification, anti-inflammatory and antioxidant effects of mahwang-tang decoction according to the storage temperature and period. Kor J Pharmacogn 2018;49(2):172–81.
44. Seo CS, Shin HK. Quantitative determination of twelve bioactive components in mahwang-tang, a traditional herbal prescription, using an LC-MS/MS. Yakhak Hoeji 2016;60(6):320–6.
45. Song MY, Kim HJ, Lee MJ. The safety guidelines for use of ma-huang in obesity treatment. Journal of Korean Oriental Association for Study of Obesity 2006;6(2):17–27.
46. Tyler VE. Herbs of choice: The therapeutic use of phytomedicinals New York: Pharmaceutical procucts press; 1994. p. 88–90.
47. Tong JY, Chao J, Dai YT, Fan ZQ, Wang DD, Qin XM, et al. Establish quality evaluation system for standard ephedrae herba decoction. Zhongguo Zhong Yao Za Zhi 2017;Mar. 42(5):823–9.
48. Aghdasi M, Bojnoordi MM, Mianabadi M, Nadaf M. Chemical components of the Ephedra major from Iran. Nat Prod Res 2016;30(3):369–71.
49. Herbalism textbook compilation committee. Herbalism 2nd edth ed. Seoul: Young Lim Press; 2011. p. 231–3.
p. 289–90.
p. 313–4.
p. 392–5.
p. 645–7.
p. 677–8.
p. 726.
50. Nahas GG. Marijuana in science and medicine New York: Raven Press; 1984. p. 25.
51. Bosy TZ, Cole KA. Consumption and quantitation of delta9-tetrahydrocannabinol in commercially available hemp seed oil products. J Anal Toxicol 2000;Oct. 24(7):562–6.
52. Cooper ZD, Haney M. Actions of delta-9 -tetrahydrocannabinol in cannabis: Relation to use, abuse, dependence. Int Rev Psychiatry 2009;Apr. 21(2):104–12.
53. Katzung BG, Masters SB, Tevor AJ. Basic and clinical pharmacology 12th edth ed. Seoul: Panmun; 2014. 42p. 587.
54. Ware MA, Jensen D, Barrette A, Vernec A, Derman W. Cannabis and the health and performance of the elite athlete. Clin J Sport Med 2018;Sep. 28(5):480–4.
55. Hilderbrand RL. Hemp & cannabidiol: What is a medicine? Mo Med 2018;Jul–Aug. 115(4):306–9.
56. Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Δ9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend 2009;Nov. 1. 105(1–2):24–32.
57. Ellis GM Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther 1985;Nov. 38(5):572–8.
58. Campos DR, Yonamine M, de Moraes Moreau RL. Marijuana as doping in sports. Sports Med 2003;33(6):395–9.
59. Brenneisen R, Meyer P, Chtioui H, Saugy M, Kamber M. Plasma and urine profiles of Δ9-tetrahydrocannabinol and its metabolites 11-hydroxy-Δ9-tetrahydrocannabinol and 11-nor -9-carboxy-Δ9-tetrahydrocannabinol after cannabis smoking by male volunteers to estimate recent consumption by athletes. Anal Bioanal Chem 2010;Apr. 396(7):2493–502.
60. Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Pharmacokinetic properties of Δ9-tetrahydrocannabinol in oral fluid of occasional and chronic users. J Anal Toxicol 2010;May. 34(4):216–21.
61. Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of Δ9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol 2007;Jun. 31(5):288–93.
62. Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, et al. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology (Berl) 2008;Dec. 201(2):171–81.
63. Hunault CC, van Eijkeren JC, Mensinga TT, de Vries I, Leenders ME, Meulenbelt J. Disposition of smoked cannabis with high Δ9-tetrahydrocannabinol content: A kinetic model. Toxicol Appl Pharmacol 2010;Aug. 1. 246(3):148–53.
64. Zuurman L, Roy C, Schoemaker RC, Hazekamp A, den Hartigh J, Bender JC, et al. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol 2008;Sep. 22(7):707–16.
65. Pacifici R, Pichini S, Pellegrini M, Tittarelli R, Pantano F, Mannocchi G, et al. Determination of cannabinoids in oral fluid and urine of “light cannabis” consumers: A pilot study. Clin Chem Lab Med 2018;Dec. 19. 57(2):238–43.
66. Lemberger L, Tamarkin NR, Axelrod J, Kopin IJ. Delta-9-tetrahydrocannabinol: Metabolism and disposition in long-term marihuana smokers. Science 1971;Jul. 2. 173(3991):72–4.
68. Gu ZP, Zhang SM, Wang CL, Lian WY, Xiao PG, Chen JM. Determation of strychnine and brucine in strychnos by HPLC. Yao Xue Xue Bao 1997;Oct. 32(10):791–4.
69. Duverneuil C, de la Grandmaison GL, de Mazancourt P, Alvarez JC. Liquid chromatography/ photodiode array detection for determination of strychnine in blood: A fatal case report. Forensic Sci Int 2004;Apr. 20. 141(1):17–21.
71. Gossel TA, Bricker JD, eds. Principles of clinical toxicology 3rd edth ed. New York: Raven Press; 1994. p. 351.
72. Goodman LS, Gilman AG. Goodman and gilman’s the pharmacological basis of therapeutics New York: Macmillan Publishing & Co., Inc; 1985. p. 582–4.
73. Ellenhorn MJ, Schonwald S, Ordog G, Wasserberger J. Ellenhorn’s medical toxicology: Diagnosis and treatment of human poisoning 2nd edth ed. Baltimore, MD: Williams and Wilkins; 1997. p. 1660.
74. Palatnick W, Meatherall R, Sitar D, Tenenbein M. Toxicokinetics of acute strychnine poisoning. J Toxicol Clin Toxicol 1997;35(6):617–20.
75. Edmunds M, Sheehan TM, Van’t Hoff W. Strychnine poisoning: Clinical and toxicological observations on a non-fatal case. J Toxicol Clin Toxicol 1986;24(3):245–55.
76. Wood DM, Webster E, Martinez D, Dargan PI, Jones AL. Case report: Survival after deliberate strychnine self-poisoning, with toxicokinetic data. Crit Care 2002;Oct. 6(5):456–9.
77. Gupta RC, Patocka J. Handbook of toxicology of chemical warfare agents London: Academic Press; 2009. p. 199.
78. Van Eenoo P, Deventer K, Roels K, Delbeke FT. Quantitative LC–MS determination of strychnine in urine after ingestion of a strychnos nux-vomica preparation and its consequences in doping control. Forensic Sci Int 2006;Dec. 20. 164(2–3):159–63.
79. Li J, Jiang Y. Rapid and sensitive determination of strychnine and brucine in human urine by capillary electrophoresis with field–amplified sample stacking. Biomed Chromatogr 2010;Feb. 24(2):186–94.
80. Kong HY, Zhou W, Guo PH, Sang ZC, Wu GN, Chen YJ, et al. Safety of individual medication of Ma Qian Zi (semen strychni) based upon assessment of therapeutic effects of Guo’s therapy against moderate fluorosis of bone. J Tradit Chin Med 2011;Dec. 31(4):297–302.
81. Xu T, Zhang Y, Yu J. HPLC determination of strychnine in nux vomica powder. Heilongjiang Medicine Journal 2012;25(1):11–2.
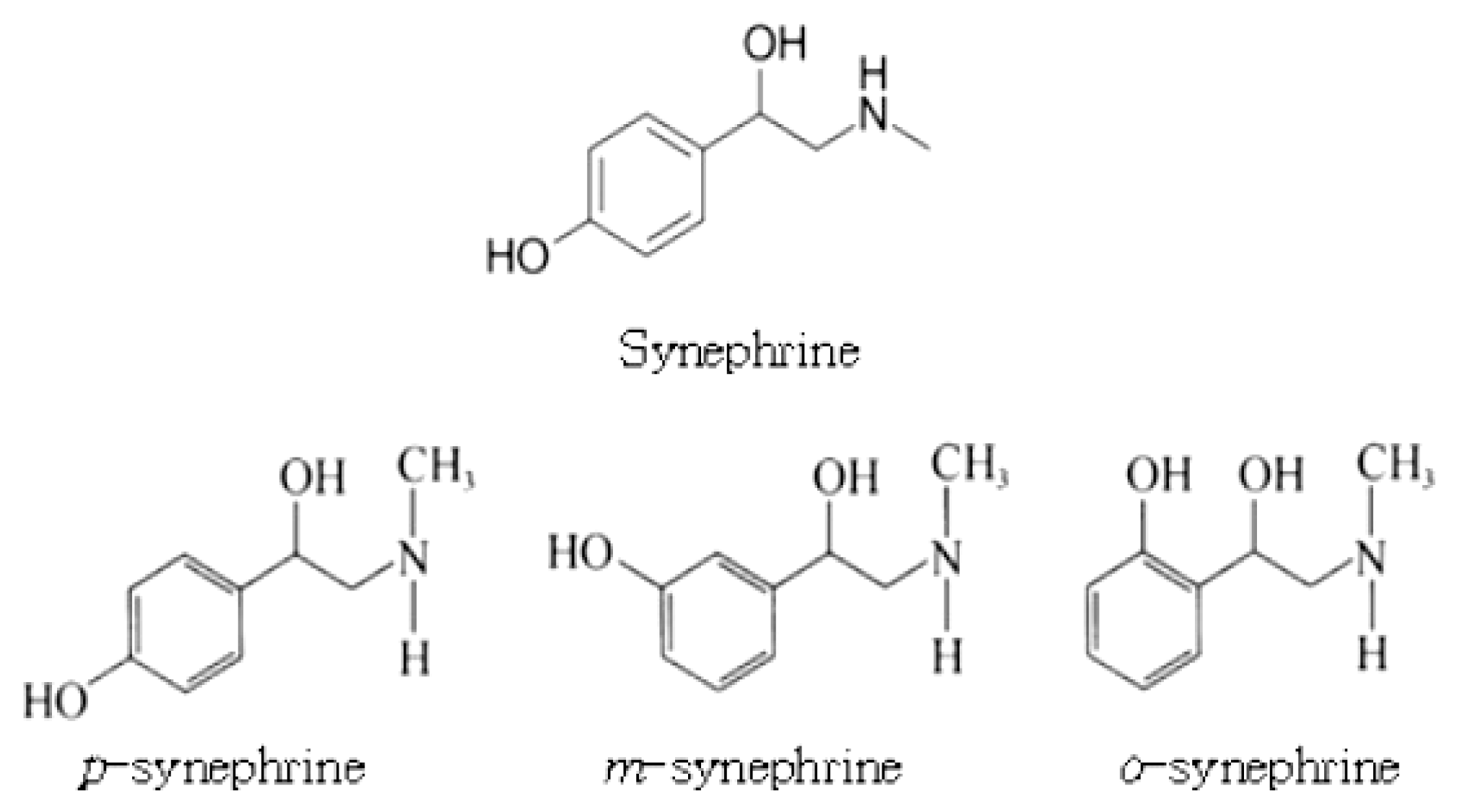
82. Tang HB, Zhang LJ, Li XL, Sun CF, Ma PJ. The experimental study of diferent solvent extracting total alkaloid from semen strychni. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2009;15(11):52–3.
83. Hu TG. Impact of traditional chinese medicine processing on physicochemical properties and efficacy of herbs. E–J Transl Med 2015;2(4):115–6.
84. Xu WF, Zhang BG, Li M, Liu GH. Determination of ephedrine in rhizoma pinelliae, rhizoma typhonii flagelliformis and their processed products by HPLC. LISHIZHEN MEDICINE AND MATERIA MEDICA RESEARCH 2007;18(4):884–5.
85. Qi HH, Wu JL, Xiang LY, Duan GL. Determination of ephedrine hydrochloride and pseudoephedrine hydrochloride in rhizoma pinelliae granules by HPLC. Science and Technology Innovation Herald 2008;Aug. 12. 16:2. (col. 1).
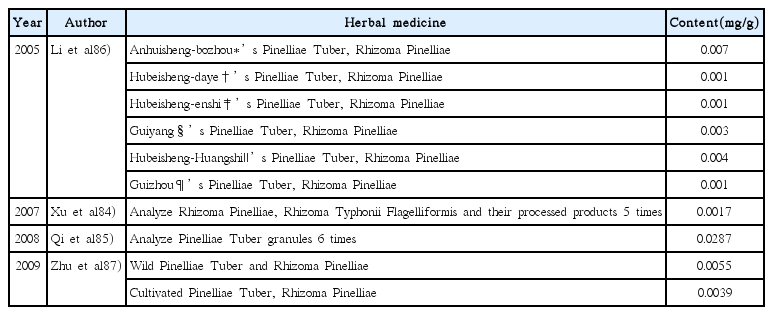
86. Li ZH, Nie J, Ni KY, Du GH. Comparison of raizoma pinelliae from different producing areas. JOURNAL OF ANALYTICAL SCIENCE 2005;21(4):393–5.
87. Zhu JJ, Guo ZY, Zhou Y, Yang J, Wang JZ, Zou K. Determination of alkaloids and the ephedrine in wild and tissue culture pinellia ternata(thunb) breit. Paper presented at: Seminar on the development and protection of Chinese herbal medicine and natural product resources; 2009 Sep 13; China Association for Pharmaceuticals and Medical Devices Technology Exchange(CPDE); Guilin, China:
88. Wu H, Tan X, Cai B, Ye D. Effect of ginger-processing on l-ephedrine contents in rhizoma pinelliae. Zhongguo Zhong Yao Za Zhi 1996;Mar. 21(3):157. :158. :190.
89. Jeong JG. A herbological study on the plants of papaveraceae in Korea. Kor J Herbol 2016;31(5):63–9.
90. Choe S, Kim S, Lee C, Yang W, Park Y, Choi H, et al. Species identification of Papaver by metabolite profiling. Forensic Sci Int 2011;Sep. 10. 211(1–3):51–60.
91. Sárközi Á, Janicsak G, Kursinszki L, Kery A. Alkaloid composition of Chelidonium majus L. studied by different chromatographic techniques. Chromatographia 2006;63(13):S81–6.
92. State Administration of Traditional Chinese Medicine. Chinese herbal medicine 3Beijing: Shanghai Science and Technology Press; 1999. p. 616–9.
93. Xinwenfeng Publishing Editorial Department. New chinese medicine dictionary Taipei: Xinwenfeng Publishing Co., Ltd; 1981. p. 605–6.
94. Fu Z, Fan X, Wang X, Gao X. Cistanches herba: An overview of its chemistry, pharmacology, and pharmacokinetics property. J Ethnopharmacol 2018;Jun. 12. 219:233–47.
95. Koehler K, Thevis M, Schaenzer W. Meta–analysis: Effects of glycerol administration on plasma volume, haemoglobin, and haematocrit. Drug Test Anal 2013;Nov–Dec. 5(11–12):896–9.
96. Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean–Chaney S, Allison DB.
Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: An update. Obes Rev 2006;Feb. 7(1):79–88.
97. Stohs SJ, Preuss HG, Shara M. A review of the receptor-binding properties of p-synephrine as related to its pharmacological effects. Oxid Med Cell Longev 2011;2011482973.
98. Gutiérrez-Hellín J, Salinero JJ, Abían-Vicen J, Areces F, Lara B, Gallo C, et al. Acute consumption of p-synephrine does not enhance performance in sprint athletes. Appl Physiol Nutr Metab 2016;Jan. 41(1):63–9.
99. Kaats GR, Miller H, Preuss HG, Stohs SJ. A 60 day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem Toxicol 2013;May. 55:358–62.
100. Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int J Med Sci 2012;9(7):527–38.
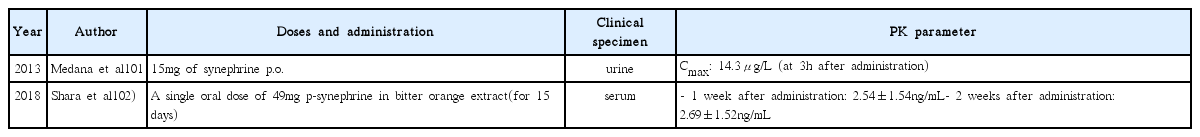
101. Medana C, Calza P, Giancotti V, Dal Bello F, Aragno M, Baiocchi C. Study of the photocatalytic transformation of synephrine: A biogenic amine relevant in anti-doping analysis. Anal Bioanal Chem 2013;Jan. 405(2–3):1105–13.
102. Shara M, Stohs SJ, Smadi MM. Safety evaluation of p–synephrine following 15 days of oral administration to healthy subjects: A clinical study. Phytother Res 2018;Jan. 32(1):125–31.
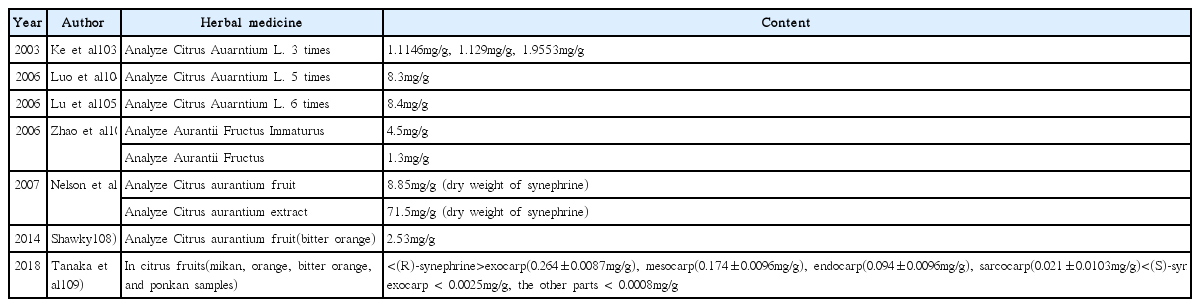
103. Ke X, Liu J, Chen C. HPLC fa ce ding cang zuo pen bi ye ji zuo shi yao cai zhong xin fu lin de han liang. Traditional Chinese Drug Research and Clinical Pharmacology 2003;14(1):45–6.
104. Luo Z, Jing H. Content determination of synephrine in citrus auarntium L. by reversed phase ion-pair HPLC. China Pharmacy 2006;17(19):1502–3.
105. Lu XF, Wang RJ, Peng GP, Deng YT. Determination of the content of synephrine in fructus aurantii immaturus by packed-column supercritical fluid chromatography. Liaoning Journal of Traditional Chinese Medicine 2006;33(2):222–3.
106. Zhao Y, Xie PS, Lu PH, Wang XH, Yang H. Determination of synephrine in Fructus aurantii immatus, Fructus aurantii, Pericarpium citri reticulatae viride and Pericarpium citri reticulatae. World Science and Technology/ Modernization of Traditional Chinese Medicine and Materia Medica 2006;8(4):64. :67. :106.
107. Nelson BC, Putzbach K, Sharpless KE, Sander LC. Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange (Citrus aurantium). J Agric Food Chem 2007;Nov. 28. 55(24):9769–75.
108. Shawky E. Determination of synephrine and octopamine in bitter orange peel by HPTLC with densitometry. J Chromatogr Sci 2014;Sep. 52(8):899–904.
109. Tanaka S, Sekiguchi M, Yamamoto A, Aizawa S, Sato K, Taga A, et al. Separation of synephrine enantiomers in citrus fruits by a reversed phase HPLC after chiral precolumn derivatization. Anal Sci [Internet] 2018. Dec. 14. [cited 2018 Dec 15]:[about 27 p.]. Available from:
https://doi.org/10.2116/analsci.18P441
. 10.2116/analsci.18P441. 30555107.
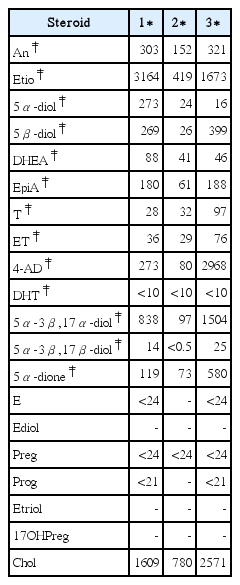
110. Wang J, He Y, Liu X, Yang Z, Yang W. Steroid profile and IRMS analysis of musk administration for doping control. Drug Test Anal 2017;Nov. 9(11–12):1779–87.
111. Thevis M, Schanzer W, Geyer H, Thieme D, Grosse J, Rautenberg C, et al. Traditional Chinese medicine and sports drug testing: Identification of natural steroid administration in doping control urine samples resulting from musk (pod) extracts. Br J Sports Med 2013;Jan. 47(2):109–14.
112. He Y, Wang J, Liu X, Xu Y, He Z. Influences of musk administration on the doping test. Steroids 2013;Nov. 78(11):1047–52.
113. Zhang HL. Extracts from plastrum testudinis (PTD) promote proliferation of rat mesenchymal stem cell(MSCs) through BMP/ID pathway [dissertation] Guangzhou: Guangzhou university of chinese medicine; 2009.
114. State Administration of Traditional Chinese Medicine. Chinese herbal medicine 9Beijing: Shanghai Science and Technology Press; 1999. p. 536–40.
p. 593–5.
115. Granados J, Gillum TL, Christmas KM, Kuennen MR. Prohormone supplement 3b-hydroxy-5a-androst-1-en-17-one enhances resistance training gains but impairs user health. J Appl Physiol (1985) 2014;Mar. 1. 116(5):560–9.
116. SLAUNWHITE WR Jr, SANDBERG AA. Metabolism of 4-C14–testosterone in human subjects III. Fate of androsterone and etiocholanolone. J Clin Endocrinol Metab 1958;Oct. 18(10):1056–66.
117. Institute of pharmacopuncturology. Pharmacopuncturology Seoul: Elsevier Korea L.L.C; 2008. p. 200–7.
118. Lee SK, Lee JD, Koh HK. The study on the Hominis Placenta aqua-acupuncture solution. The Journal of Korea Acupuncture & Moxibustion Society 2000;17(1):67–74.
120. Melsmon [Internet] Tokyo: Millennia Concepts (Official Website); c2015. [cited 2018 Nov 29]. Available from:
http://melsmon.com/
.
121. Tsukiyama M, Ueki T, Yasuda Y, Kikuchi H, Akaishi T, Okumura H, et al.
B2-adrenoceptor -mediated tracheal relaxation induced by higenamine from Nandina domestica
. Thunberg Planta Med 2009;Oct. 75(13):1393–9.
122. Kashiwada Y, Aoshima A, Ikeshiro Y, Chen YP, Furukawa H, Itoigawa M, et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorg Med Chem 2005;Jan. 17. 13(2):443–8.
123. Chang YC, Chang FR, Khalil AT, Hsieh PW, Wu YC. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana
. Z Naturforsch C 2003;Jul–Aug. 58(7–8):521–6.
124. Kimura I, Chui LH, Fujitani K, Kikuchi T, Kimura M. Inotropic effects of (±)-higenamine and its chemically related components, ( )-R- coclaurine and ( )-S-reticuline, contained in the traditional sino-japanese medicines “Bushi” and “Shin-i” in isolated guinea pig papillary muscle. Jpn J Pharmacol 1989;May. 50(1):75–8.
125. Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, Zhu YY. Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2 -adrenergic receptor agonist. Acta Pharmacol Sin 2008;Oct. 29(10):1187–94.
126. Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC, Yun-Choi HS, et al. Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root. J Pharmacol Exp Ther 1999;Oct. 291(1):314–20.
127. Praman S, Mulvany MJ, Williams DE, Andersen RJ, Jansakul C. Hypotensive and cardio-chronotropic constituents of Tinospora crispa and mechanisms of action on the cardiovascular system in anesthetized rats. J Ethnopharmacol 2012;Mar. 6. 140(1):166–78.
128. Minami H, Dubouzet E, Iwasa K, Sato F. Functional analysis of norcoclaurine synthase in Coptis japonica
. J Biol Chem 2007;Mar. 2. 282(9):6274–82.
129. Zhang N, Lian Z, Peng X, Li Z, Zhu H. Applications of higenamine in pharmacology and medicine. J Ethnopharmacol 2017;Jan. 20. 196:242–52.
130. Lee SR, Schriefer JM, Gunnels TA, Harvey IC, Bloomer RJ. Acute oral intake of a higenamine-based dietary supplement increases circulating free fatty acids and energy expenditure in human subjects. Lipids Health Dis 2013;Oct. 21. 12:148.
131. Feng S, Jiang J, Hu P, Zhang JY, Liu T, Zhao Q, et al. A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy chinese subjects. Acta Pharmacol Sin 2012;Nov. 33(11):1353–8.
132. Okano M, Sato M, Kageyama S. Determination of higenamine and coclaurine levels in human urine after the administration of a throat lozenge containing Nandina domestica fruit. Drug Test Anal 2017;Nov. 9(11–12):1788–93.
133. Du YR, Li F, Xu RY, Zhang Y, Ouyang M, Jing HL. Tolerability of higenamine hydrochloride in healthy volunteers. Chin J Clin Pharmacol 2007;23(4):125–60.
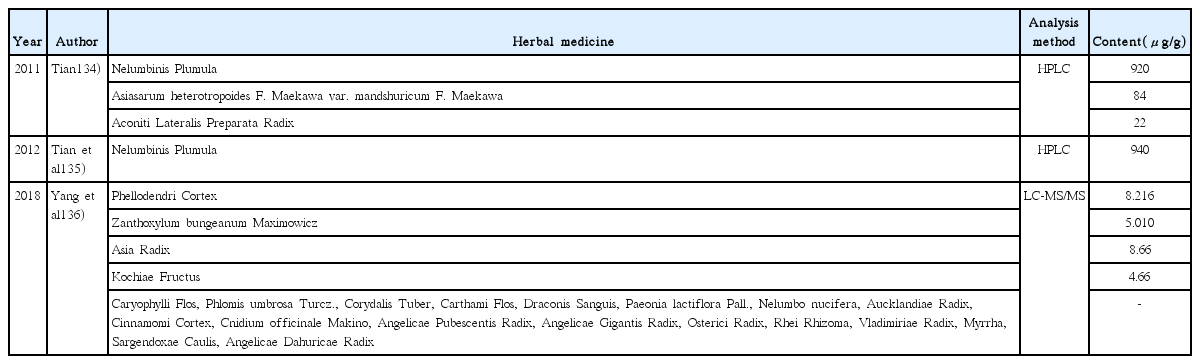
134. Tian HF. Determination of higenamine by reversed phase high performance liquid chromatography with precolumn derivatization [dissertation] Yanji city: Yanbian university; 2011.
135. Tian HF, Yi T, Jing DR. Determination of higenamine in Nelumbo nucifera by HPLC with precolumn derivatization. Journal of Yanbian University(Natural Sciene) 2012;38(2):150–3.
136. Yang M, He S, Zhang D, Sun B, Wang Y. Study on determination of higenamine in chinese medicinal materials by LC-MS/MS. China measurement & test 2018;44(3):61–5.
137. Kim SY, Geum DH, Lee MJ. A study on doping test of Bojungchisheup-tang
. Korean J Ori Med 1997;3(1):289–319.
138. Yoo HJ. Verification of efficacy as an ergogenic aid and safety in doping of Sibjeondaebo-tang [dissertation] Seoul: Kyunghee university; 2014.
139. Lee JH. SK lim seok jin detection of doping...36 games ‘suspension’. The Kyunghyang Shinmun 2017;Oct. 28. Sect A:24. (col. 6).
140. Kim HJ, Kwak BM, Ahn JH, Park JS. Simultaneous determination of synephrine and N-methyltyramine in orange fruit and juice from korean market by UPLC-FLD. Korean J Food Sci Technol 2014;46(3):276–82.
141. Balasubramanian J, Maraicar KSH, Ananth DB, Vijayakumar N, Dhanalakishmi R. DHEA: The remedy for andropause. Indian J Med Healthcare 2012;May. 1(2):25–31.
142. Son YI. Verification and verification, seeking 100% safety : The placenta, which has been processed for 11 weeks at the greencross placenta injection plant in chungbuk eumseong, has been turned into a medicine. Weekly Donga 2009;Oct. 27. 708:36–9.