Usage of informed consent form for Bee-venom pharmacopuncture Therapy at korean medicine hospitals and Proposal for development of a standard informed consent form
Article information
Abstract
Objectives
We investigated the current status of the consent form for bee-venom pharmacopuncture therapy, which is using in Korean medicine hospitals. We suggest the development of a standard informed consent form.
Method
Through the questionnaire survey, status of using informed consent form was identified at 24 Korean medicine hospitals. We analyze different types of informed consent form, which was developed by each hospitals. We investigated the types of informed consent forms for various medical procedures through electronic searches. A standard informed consent form for bee-venom pharmacopuncture therapy was developed based on the medical law and the standard informed consent form for medical procedures developed by Korea Fair Trade Mediation Agency.
Result
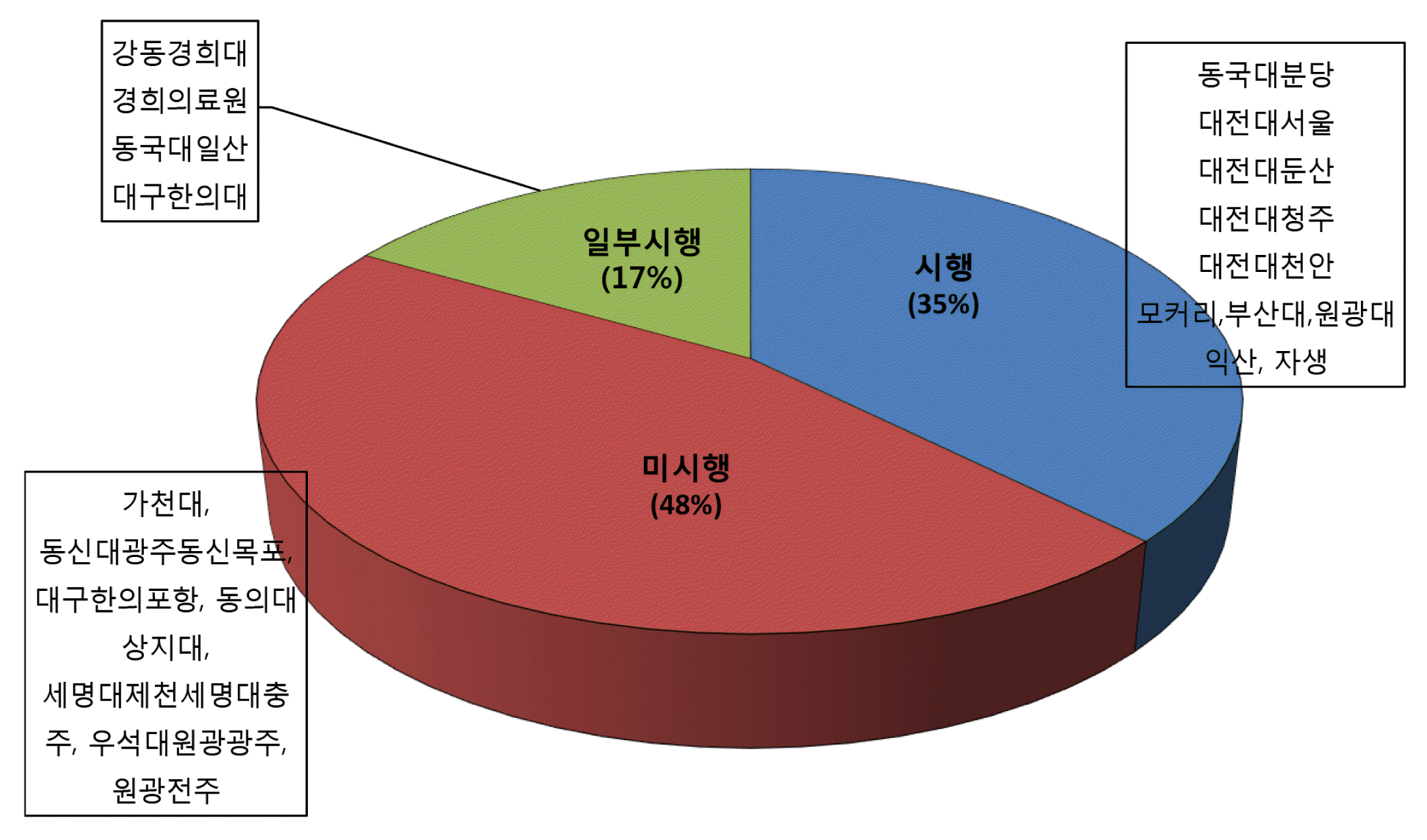
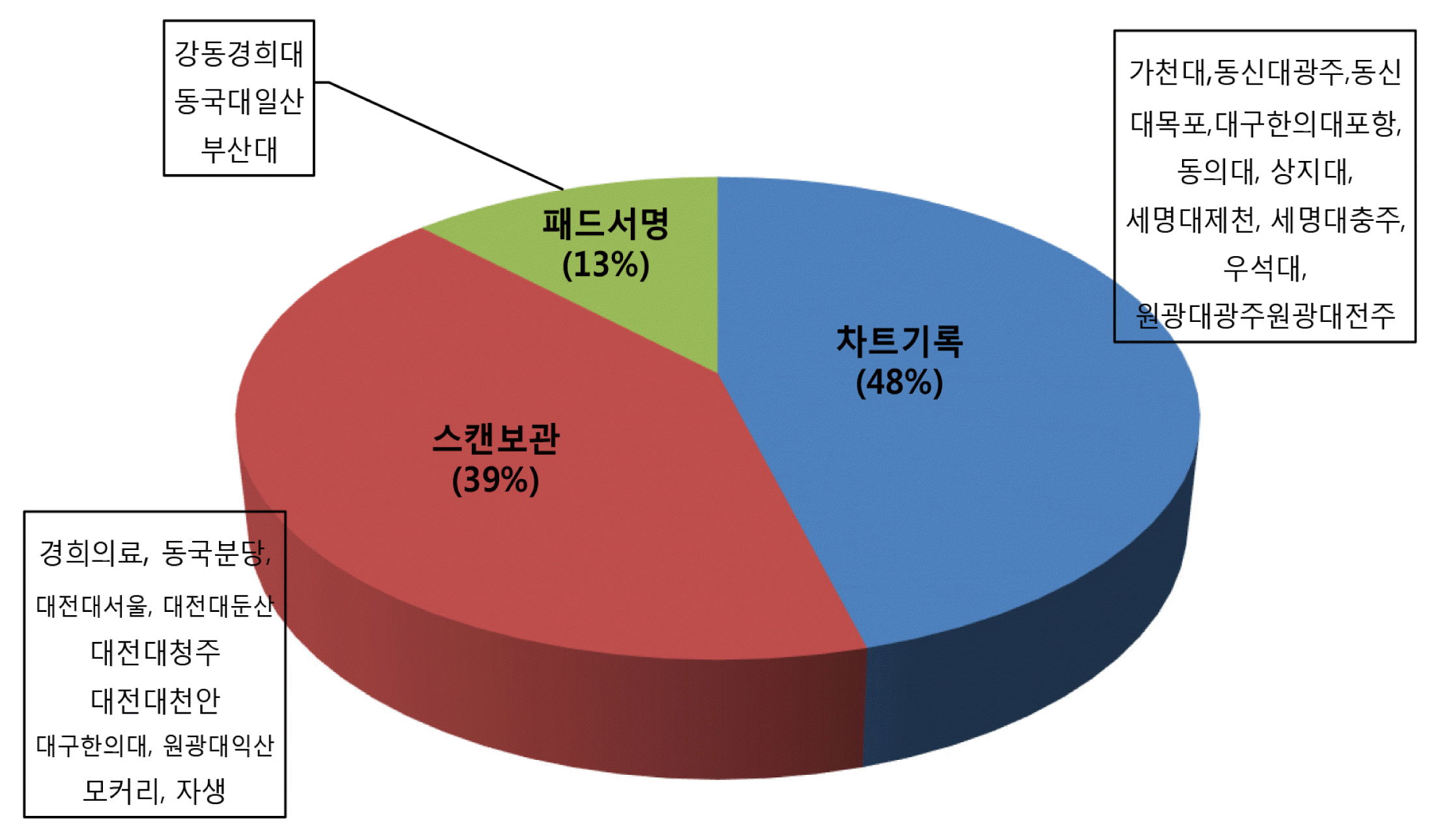
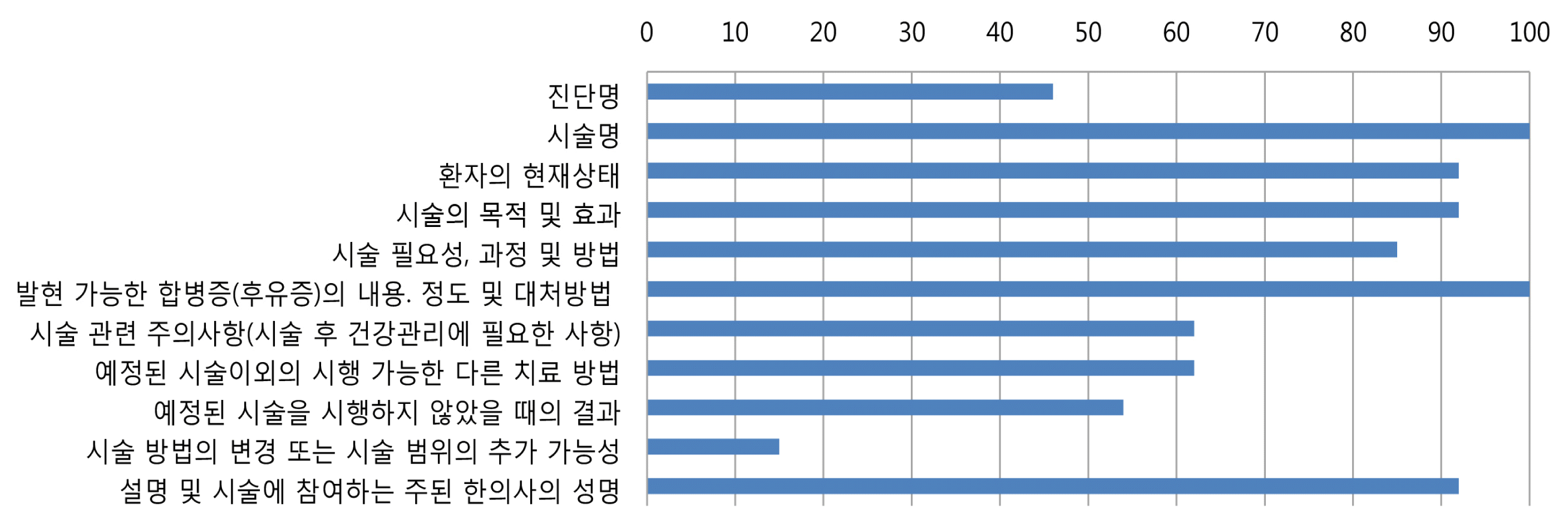
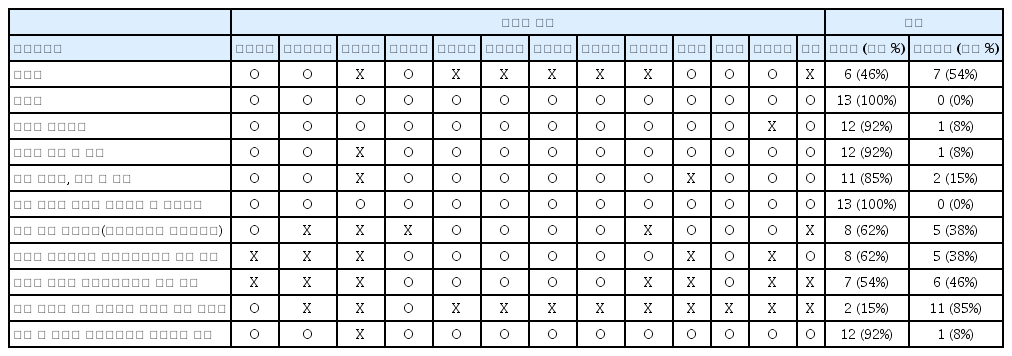
In our survey, 65% of the hospitals do not use consent well, only 35% of the hospitals use informed consent form, and the most hospitals use self-developed informed consent form. As a result of analyzing the contents of informed consent form used in each hospitals, the explanation of diagnosis, treatment precautions, suggestions for other treatments, consequences of not performing the scheduled procedure, possibility of treatment change was insufficient. 48% of hospitals manage consent in recording on a chart, 39% in scanned documents, and 13% in digital electronic consent form.
Conclusion
A standard informed consent form for Bee-venom pharmacopuncture therapy include diagnosis, effectivness, necessity, indications, method, skin reaction test, hypersensitivity questionnaire, treatment precautions, possible hypersensitivity reactions and countermeasures, suggestions for other treatments, consequences of not performing the scheduled procedure, possibility of treatment change and the name of doctor.