Effect of 2.5 hr-interval single oral combination treatment of Gamisoyo-san with Tamoxifen on the pharmacokinetics profiles of Tamoxifen in rats
Article information
Abstract
Objectives
The object of this study was to elucidate the possible effects on the pharmacokinetics of tamoxifen after single oral co-administration of Gamisoyo-san (GMSYS) with 2.5 hr-intervals combination therapy of tamoxifen with GMSYS.
Methods
After 2.5 hr of 50 mg/kg of tamoxifen treatment, GMSYS 100 mg/kg was administered. The plasma was collected at 30 min before administration, 30 min, 1, 2, 3, 4, 6, 8 and 24 hrs after end of GMSYS treatment, and plasma concentrations of tamoxifen were analyzed using LC-MS/MS methods. PK parameters of tamoxifen were analysis as compared with tamoxifen single administered rats.
Results
Although single co-administration with GMSYS with 2.5 hr-interval induced increased trends of plasma tamoxifen concentrations, there are no significant changes on the plasma concentrations of tamoxifen were demonstrated in tamoxifen and GMSYS 100 mg/kg co-administrated rats with 2.5 hr-intervals as compared to those of tamoxifen single 50 mg/kg treated rats, and also GMSYS co-administrated rats did not showed any significant changes on the all pharmacokinetic parameters as compared to those of tamoxifen single formula treated rats.
Conclusions
According to the this study, single co-administration of GMSYS with 2.5 hr-intervals did not critically influenced on the oral bioavailability of tamoxifen, suggesting GMSYS did not critically influenced on the absorption and excretion of tamoxifen, the oral bioavailability, when they were co-administered with 2.5 hr-intervals, at the dose levels of tamoxifen 50 mg/kg and GMSYS 100 mg/kg.
Introduction
Tamoxifen (NolvadexTM) is contraindicated, when used in women with ductal carcinoma in situ and women at high risk for breast cancer, concurrent anticoagulant therapy with a warfarin derivative1), and should be used with caution in patients with leukopenia or thrombocytopenia2) and pregnant34). Hot flashes, vaginal discharge, menstrual irregularities and weight loss are common side effects related with tamoxifen treatment35). As results of combination therapies with other drugs to improve the side effects of tamoxifen or to achieve synergic effects, various drug-drug interactions of tamoxifen have been evaluated; Because tamoxifen was metabolized by a substrate of CYP3A, 2C9, 2D66), it interacted with various drugs, namely, combinations containing any of the following medications, depending on the amount present, may also interact with aminoglutethimide - decreased plasma tamoxifen and N-desmethyltamoxifen concentrations7), anticoagulants - enhanced warfarin effects8), bromocriptine - increased plasma tamoxifen and N-desmethyltamoxifen concentrations9), letrozole - decreased plasma letrozole concentrationsa10), medroxyprogesterone - decreased plasma N-desmethyltamoxifen concentrations but did not reduce plasma tamoxifen concentrations11), phenobarbital - decreased plasma tamoxifen concentrations12), rifampin - decreased plasma tamoxifen and N-desmethyltamoxifen concentrations13), and cyclosporine, erythromycin, diltiazem, erythromycin and nifedipine - competitively inhibited formation of N-desmethyltamoxifen in vitro14), respectively. However, interactions with herbal products have not been established except for some restricted natural compounds; tamoxifen enhanced warfarin effects, and it is contraindicate that co-administration of tamoxifen and wafarin8).
Gamisoyo-san (GMSYS), one of the commonly prescribed herbal formulas consisted of 10 herbs - Angelicae Gigantis Radix, Paeoniae Radix, Atractylodis Rhizoma Alba, Hoelen, Bupleuri Radix, Gardeniae Fructus, Glycyrrhizae Radix et Rhizoma, Moutan Cortex, Menthae Herba and Zingiberis Rhizoma, has routinely been prescribed to relieve irregularity of menstruation, anxiety associated with the menstrual cycle and various menopause-related symptoms occurring in climacteric disturbance15), Thus, it seems reasonable to suggest that GMSYS should be considered an alternative to hormone-replacement-therapy for patients with climacteric symptoms, especially those who presented psychological symptoms16). It is often prescribed for women who complain of general fatigue, hot flushes, stiff shoulders, insomnia, diaphoresis, depression, irritability, etc. It is also applied to treat diverse diseases, including autonomic imbalance, neurosis, eruption, chloasma (melasma), alopecia, constipation17), functional dyspepsia18), Parkinson's disease and convulsions19). Although single co-administration with GMSYS within 5 min did not influenced on the pharmacokinetic parameters of oral tamoxifen, GMSYS significantly increased the oral absorption of tamoxifen (p<0.05), when they were single co-administered within 5 min20). It, therefore, the effects of GMSYS co-administration with reasonable intervals, 2.5 hrs, on the pharmacokinetics of tamoxifen were observed as a process of the comprehensive and integrative medicine, combination therapy of tamoxifen with GMSYS to achieve synergic pharmacodynamics and reduce toxicity in breast cancer.
Materials & methods
1. Animals and husbandry
A total of eighty-eight male SPF.VAF Outbred Crl:CD [Sprague-Dawley (SD)] rats (Seungnam, Korea) were used after acclimatization for 16 days. Animals were allocated five per polycarbonate cage in a temperature (20–25 °C) and humidity (40–45%) controlled room. Light : dark cycle was 12 hr : 12 hr and feed (Samyang, Korea) and water were supplied free to access. After sixteen days of acclimatization, five rats per group were selected based on the body weights. All animals were marked by picric acid, and overnight fasted (about 18 hrs; water was not restricted) before treatment, and further fasted during 3 hrs after end of treatment. Animal experiments were conducted according to the national regulations of the usage and welfare of laboratory animals, and approved by the Institutional Animal Care and Use Committee in Daegu Haany University (Gyeongsan, Gyeongbuk, Korea) [Approval No DHU2013-058].
2. Test articles and formulation
Light brown granules of GMSYS (HANPOONG PHARM & FOODS Co, Ltd., Seoul, Korea), produced according to Korean Good Manufacturing Practice and permitted and regulated by the Korean Food & Drug Administration (Seoul, Korea) were used in this experiment, and tamoxifen (Hangzhou Tacon Co., Ltd, Hangzhou, China) was used as control drug. Both samples are well dissolved (up to 20 mg/ml solutions in GMSYS and upto 10 mg/ml solutions in tamoxifen) in distilled water as vehicle, respectively.
3. Groupings and administration
Five rats per group (two groups) were used in this study as follows. The doses of test materials were selected based on our previous pharmacokinetics study after single co-administration of tamoxifen and GMSYS within 5 min 20), the doses of test materials were selected based on their toxicity and pharmacodynamics – 50 mg/kg of tamoxifen with 100 mg/kg of GMSYS. Two hrs thirty min after 50 mg/kg of tamoxifen treatment, GMSYS 100 mg/kg was orally administered. In tamoxifen single treated rats, 50 mg/kg of tamoxifen was orally administered, and then distilled water 5 ml/kg was orally administered after 2.5 hr, instead of GMSYS. Each tamoxifen or GMSYS was single orally administered, in a volume of 5 ml/kg, dissolved in distilled water.
4. Plasma collections
All rats were anesthetized with 2 to 3% isoflurane (Hana Pharm. Co., Hwasung, Korea) in the mixture of 70% N2O and 28.5% O2, and blood samples (0.5 ml) were collected into 50 IU heparinized tubes via the orbital plexus at 30 min before treatment (as a control), 30 min, 1, 2, 3, 4, 6, 8 and 24 hrs after end of oral administration. Blood samples were immediately centrifuged for 10 min at 13,000 rpm and about 0.3 ml aliquots of plasma were stored in a −150 °C deep freezer until analysis of tamoxifen.
5. Sample preparation and calibrations
Primary stock solution, 1.0 mg/ml of tamoxifen in 100% MeOH (Baker, Phillipsburg, NJ, USA) and internal standard working solution, carbamazepine (Sigma-Aldrich, Sigma, St. Louise, MO, USA) 500 ng/ml in acetonitrile were prepared. Working standard solutions were prepared by dilution with acetonitrile. All standard solutions were stored at −20 °C in the dark when not in use, and calibrated the standard samples as 100 μl of blank plasma; working standard solutions and internal standard working solution were mixed with 200 μl of acetonitrile. In addition, 100 μl of sample plasma and internal standard working solution were mixed with 200 μl of acetonitrile. The mixtures were mixed by vortex-mixing and centrifuged at 12,000 rpm for 10min at 4 °C. The clear supernatants (5.0 μl) were transferred to injection vials and the aliquot was injected into the LC-MS/MS system.
6. LC-MS/MS conditions
Concentrations of tamoxifen in the rat plasma samples were determined LC-MS/MS method. Chromatographic analysis was performed using an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with on-line degasser, binary pump, autosampler and column compartment. Separation of the analyte from potentially interfering material was achieved at ambient temperature using Waters SymmetryTM C18 columns (2.1×50 mm, 3.5 μm) (Waters Corp., Milford, MA, USA) at column oven 30 °C. The mobile phase used for the chromatographic separation was composed of 50% distilled water (0.1% formic acid)/50% acetonitrile, and was delivered isocratically at a flow rate of 0.35 ml/min. The column effluent was monitored using an API 2000 triple-quadruple mass-spectometric detector (Applied Biosystems, Foster City, CA, USA). The instrument was equipped with an electrospray interface in positive ion mode, and controlled by the Analyst version 1.4.1 software (Applied Biosystems, Foster City, CA, USA) (Linear (1/x2, no Iterate)). Samples were introduced to the interface through a Turbo IonSpray with the temperature set at 500 °C. A high positive voltage of 4.0 kV was applied to the ion spray. Nitrogen was used as the nebulizer gas, curtain gas, and collision gas with the settings of 70, 20, and 7, respectively. The multiple reaction monitoring (MRM) detection method was employed for the detection of tamoxifen; the transitions monitored were carbamazepine (IS): m/z 237>194 (Retention time: 0.63 min), tamoxifen: 372>178 (Retention time: 0.55 min). Calibration curves of tamoxifen were linear over the ranges studied with r2>0.999. The lower limit of quantification of the tamoxifen in the rat plasma was 8 ng/ml.
7. Pharmacokinetic analysis
The plasma concentration data were analyzed using a noncompartmental method on commercial pharmacokinetics data analyzer programs (PK solutions2.0; Summit, Montrose, CO, USA)21). The elimination rate constant (Kel) was calculated by the log-linear regression of tamoxifen concentration data during the elimination phase, and the terminal half-life (t1/2) was calculated by 0.693/Kel. The peak concentration (Cmax) and time to reach the peak concentration (Tmax) of tamoxifen in the plasma were obtained by visual inspection of the data in the concentration-time curve. The area under the plasma concentration-time curve (AUC0-t) from time zero to the time of the last measured concentration (Clast) was calculated using the linear trapezoidal rule22). The AUC zero to infinity (AUC0-inf) was obtained by adding AUC0-t and the extrapolated area was determined by Clast/Kel. The mean residence time infinity (MRTinf) was calculated by dividing the first moment of AUC (AUMC0-inf) by AUC0-inf.
8. Statistical analyses
All the means are presented with their standard deviation of five rats (Mean ± S.D. of five rat plasma concentrations of tamoxifen). The pharmacokinetic parameters were compared using a non-parametric comparison test, Mann-Whitney U (MW) test, on the SPSS for Windows (Release 14.0K, SPSS Inc., USA). A p-value <0.05 was considered statistically significant. In addition, the percent changes between tamoxifen single treated rats and tamoxifen with GMSYS 2.5 hr-interval co-administered rats were calculated to help the understanding of the effects of co-administration: Percentage changes as compared with tamoxifen 50 mg/kg single treated mice (%) = [((Data of GMSYS co-administrated rats – data of tamoxifen single treated rats)/Data of tamoxifen single treated rats) × 100].
Results
1. Changes on the plasma concentrations of tamoxifen
Tamoxifen was detected from 30 min to 24 hrs after end of administration in the both tamoxifen single or 2.5 hr-interval co-administered rats with GMSYS, respectively. Although slight increases trends of plasma concentration of tamoxifen were demonstrated throughout all blood collecting points in co-administrated rats, there are no significant changes on the plasma tamoxifen concentrations were observed after 2.5 hr-interval co-administration of GMSYS 100mg/kg and tamoxifen 50 mg/kg as compared with tamoxifen single treated rats, in the present study (Fig 1). The plasma tamoxifen concentrations at 30min, 1, 2, 3, 4, 6, 8 and 24 hrs after end of treatment were changed as 14.15, 1.07, 13.19, 23.86, 21.60, 12.95, 16.96 and 0.48% in tamoxifen + GMSYS treated rats as compared with tamoxifen single treated rats, respectively.
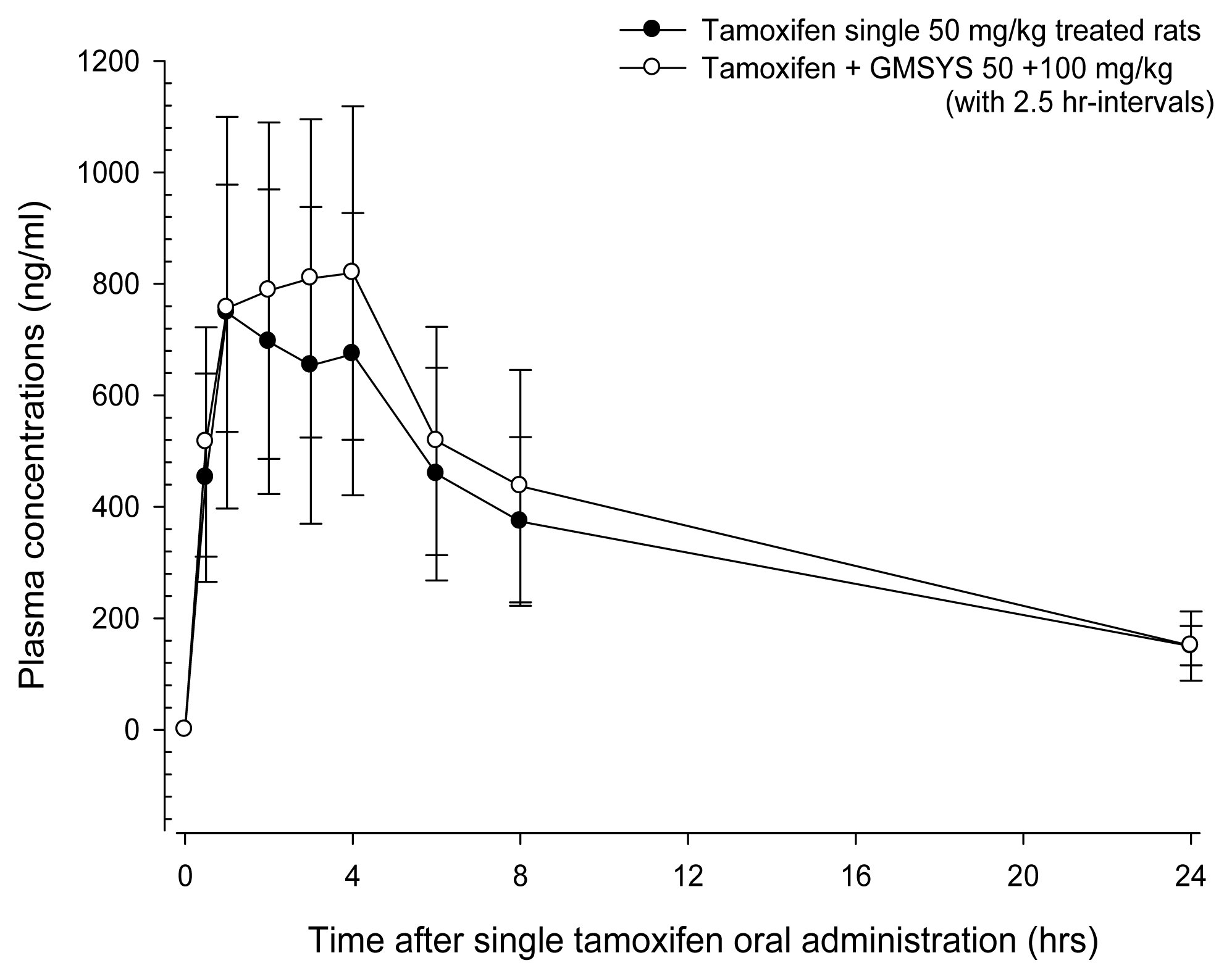
Plasma Concentrations of Tamoxifen with and without GMSYS Co-administration with 2.5hr-intervals in Male Rats. Tamoxifen was detected from 30 min to 24 hrs after end of administration in the both tamoxifen single or 2.5 hr-interval co-administered rats with GMSYS, respectively. Although slight increases trends of plasma concentration of tamoxifen were demonstrated throughout all blood collecting points in co-administrated rats, there are no significant changes on the plasma tamoxifen concentrations were observed after 2.5 hr-interval co-administration of GMSYS 100 mg/kg and tamoxifen 50 mg/kg as compared with tamoxifen single treated rats. Values are expressed as mean ± S.D. of five rats (ng/ml). GMSYS = Gamisoyo-san purchase from HANPOONG PHARM & FOODS Co, Ltd. (Seoul, Korea).
2. Changes on the Tmax of tamoxifen
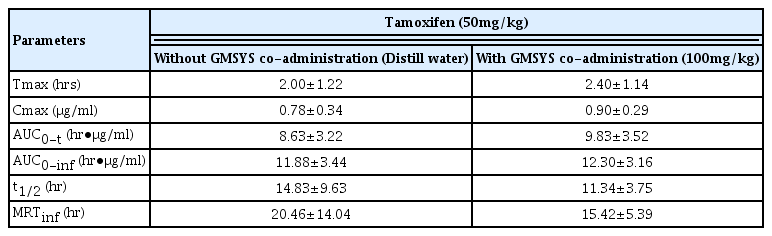
The Tmax of tamoxifen were non-significantly increased as 20.00% in 2.5 hr-interval co-administrated rats with tamoxifen 50 mg/kg and GMSYS 100 mg/kg (2.40±1.14 hr) as compared with tamoxifen single treated rats (2.00±1.22 hr) (Table 1).
3. Changes on the Cmax of tamoxifen
The Cmax of tamoxifen were non-significantly and slightly increased as 16.42% in 2.5 hr-interval co-administrated rats with tamoxifen 50 mg/kg and GMSYS 100 mg/kg (0.90±0.29 μg/ml) as compared with tamoxifen single treated rats (0.78±0.34 μg/ml) (Table 1).
4. Changes on the AUC of tamoxifen
The AUC0-t of tamoxifen were non-significantly increased as 13.89% in 2.5 hr-interval co-administrated rats with tamoxifen 50 mg/kg and GMSYS 100 mg/kg (9.83±3.52 hr•μg/ml) as compared with tamoxifen single treated rats (8.63±3.22 hr•μg/ml). In addition, AUC0-inf of tamoxifen were also non-significantly increased as 3.55% in 2.5 hr-interval co-administrated rats with tamoxifen and GMSYS (12.30±3.16 hr•μg/ml) as compared with tamoxifen single treated rats (11.88±3.44 hr•μg/ml) (Table 1).
5. Changes on the t1/2 of tamoxifen
The t1/2 of tamoxifen were non-significantly decreased as −23.53% in 2.5 hr-interval co-administrated rats with tamoxifen 50 mg/kg and GMSYS 100mg/kg (11.34±3.75 hr) as compared with tamoxifen single treated rats (14.83±9.63 hr) (Table 1).
6. Changes on the MRTinf of tamoxifen
The MRTinf of tamoxifen were non-significantly decreased as −24.65% in 2.5 hr-interval co-administrated rats with tamoxifen 50 mg/kg and GMSYS 100 mg/kg (15.42±5.39 hr) as compared with tamoxifen single treated rats (20.46±14.04 hr) (Table 1).
Discussion
Although single 2.5 hr-interval co-administration with GMSYS induced increased trends of plasma tamoxifen concentrations, there are no significant changes on the plasma concentrations of tamoxifen were demonstrated in tamoxifen and GMSYS 100 mg/kg co-administrated rats with 2.5 hr-intervals as compared to those of tamoxifen single 50 mg/kg treated rats, and also GMSYS co-administrated rats did not showed any significant changes on the all pharmacokinetic parameters as compared to those of tamoxifen single formula treated rats, in this experiment, somewhat differed from the results of single co-administration within 5 min, in which significant increases of plasma tamoxifen concentrations were noticed20). These findings are considered as direct evidences that GMSYS did not critically influenced on the absorption and excretion of tamoxifen, the oral bioavailability, when they were co-administered with 2.5 hr-intervals, at the dose levels of tamoxifen 50 mg/kg and GMSYS 100 mg/kg. Hence, 2.5 hr-interval co-administration is considered as suitable regime of GMSYS and tamoxifen to achieve synergic pharmacodynamics and reduce toxicity for breast cancer patients as comprehensive and integrative therapy, and further pharmacodynamic studies should be conducted along with tamoxifen and GMSYS 2.5 hr-interval co-administrations. However, more detail pharmacokinetic studies should be tested to conclude the effects of GMSYS on the pharmacokinetics of tamoxifen, when they were co-administered with 2.5 hr-intervals, like repeated co-administrations.
Tamoxifen was absorbed slowly following oral administration and Tmax of tamoxifen occur about 3–6 hrs after a single dose23) but it rapidly and extensively metabolized in the liver, through a substrate of CYP3A, 2C9, 2D626 including an active major metabolite, N-desmethyltamoxifen has biologic activity similar to that of the parent drug24). Steady-state concentrations of tamoxifen are attained after 3–4 weeks and those of N-desmethyltamoxifen, an active metabolite, are attained after 3–8 weeks25). Tamoxifen excreted principally in feces as polar conjugates26) with about 5–7 days of t1/2 in tamoxifen and 9–14 days in N-desmethyltamoxifen23). Clearance of tamoxifen is higher in female children 2–10 years of age than in women27). In the present study, Tmax of tamoxifen in tamoxifen single oral treated rats was detected as 2.00±1.22 hr, and Cmax, AUC0-t, AUC0-inf, t1/2 and MRTinf were detected as 0.78±0.34 μg, 8.63±3.22 hr•μg/ml, 11.88±3.44 hr•μg/ml, 14.83±9.63 hr and 20.46±14.04 hr, respectively. In tamoxifen with GMSYS co-administered rats with 2.5 hr-intervals, Tmax, Cmax, AUC0-t, AUC0-inf, t1/2 and MRTinf of tamoxifen were detected as 2.40±1.14 hr, 0.90±0.29 μg, 9.83±3.52 hr•μg/ml, 12.30±3.16 hr•μg/ml, 11.34±3.75 hr and 15.42±5.39 hr as changed as 20.00, 16.42, 13.89, 3.55, −23.53 and −24.65% as compared with tamoxifen 50 mg/kg single oral treated rats, respectively. However, no significant chamges on the pharmacokinetic parameters of oral tamoxifen were observed after single 2.5 hr-interval co-administration with GMSYS as compared with tamoxifen single formula treated rats, at dosage levels of tamoxifen 50 mg/kg and GMSYS 100 mg/kg.
Tamoxifen rapidly and extensively metabolized in the liver, through a substrate of CYP3A, 2C9, 2D626 to active major metabolite, N-desmethyltamoxifen 24) and, therefore, tamoxifen can be interacted with various drugs like aminoglutethimide7), anticoagulants8), bromocriptine9), letrozole10), medroxyprogesterone11), phenobarbital12) and rifampin13). In addition the possibilities that tamoxifen competitively interacted with cyclosporine, erythromycin, diltiazem, erythromycin and nifedipine were also suggested in vitro experiments14). The severities of various side effects arise from tamoxifen treatment, especially bone loss28), endometrial cancer29), thromboembolism30), fatty liver31), reduced cognition32), semantic memory scores33) and libido34), premature growth plate fusion35), immune suppression36) and hypersensitivity37) are considered as directly co-related with absorption and excretion of tamoxifen or pharmacodynamics. We already observed that oral co-administration of Jaeumkanghwa-tang, a traditional yin-tonifying herbal medicine has been used for various oriental obstetrical and gynecological fields within 5min did not critically influenced on the pharmacokinetics profiles of tamoxifen after single38) and repeated39) co-administration at dosage levels of 50 mg/kg in tamoxifen and 100 mg/kg in Jaeumkanghwa-tang, respectively. In this experiment, single co-administration of GMSYS with 2.5 hr-intervals did not critically influenced on the oral bioavailability of tamoxifen, quite differed from the results of single co-administration within 5 min, in which marked increases of the tamoxifen absorptions were demonstrated20), suggesting GMSYS did not critically influenced on the absorption and excretion of tamoxifen, the oral bioavailability, when they were co-administered with 2.5 hr-intervals, at the dose levels of tamoxifen 50 mg/kg and GMSYS 100 mg/kg. It, therefore, considered that 2.5 hr-interval co-administration is suitable regime of GMSYS and tamoxifen to achieve synergic pharmacodynamics and reduce toxicity for breast cancer patients as comprehensive and integrative therapy, and further pharmacodynamic studies should be conducted along with tamoxifen and GMSYS 2.5 hr-interval co-administrations. However, more detail pharmacokinetic studies should be tested to conclude the effects of GMSYS on the pharmacokinetics of tamoxifen, when they were co-administered with 2.5 hr-intervals, like repeated co-administrations.
Conclusions
Base on the results of the present study, single co-administration of GMSYS with 2.5 hr-intervals did not critically influenced on the oral bioavailability of tamoxifen, suggesting GMSYS did not critically influenced on the absorption and excretion of tamoxifen, the oral bioavailability, when they were co-administered with 2.5 hr-intervals, at the dose levels of tamoxifen 50 mg/kg and GMSYS 100 mg/kg. Hence, 2.5 hr-interval co-administration is considered as suitable regime of GMSYS and tamoxifen to achieve synergic pharmacodynamics and reduce toxicity for breast cancer patients as comprehensive and integrative therapy, and further pharmacodynamic studies should be conducted along with tamoxifen and GMSYS 2.5 hr-interval co-administrations. However, more detail pharmacokinetic studies should be tested to conclude the effects of GMSYS on the pharmacokinetics of tamoxifen, when they were co-administered with 2.5 hr-intervals, like repeated co-administrations.
Acknowledgments
This work was supported by grant of Korea of Health & Welfare, Republic of Korea (Project No: 20-11-0-090-091-3000-3033-320).