Rhus Verniciflua Stokes Extract Suppresses Expression of Metalloproteinases, iNOS and COX-2 in THP-1 Cells Via Inhibiting NF-κB and MAPK Phosphorylation
Article information
Abstract
Objectives
The aim of this study is to investigate the mechanisms involved in the anti-inflammatory and anti-tumor effects of Rhus verniciflua Stokes (RVS) on PMA-differentiated human monocytic leukemia THP-1 cells.
Methods
Cells were treated with various concentrations of RVS decoction (0–300μg/ml) for 24, 48, and 72h. Cell viability was evaluated by MTS/PMS assay. The expressions of MMP-2, MMP-9, TIMP-1, TIMP-2, iNOS and COX-2 mRNA and proteins were measured using RT-PCR and western blotting, respectively.
Results
RVS suppressed expression of MMP-2 and MMP-9 mRNA. It also down-regulated iNOS and COX-2 mRNA and protein expression. RVS inhibited NF-κB p65 activity and the phosphorylation of Akt and MAPK (ERK and p38 MAPK). Instead, the phosphorylation of JNK is increased at a very low concentration but decreased at higher concentrations.
Conclusion
RVS is regarded to inhibit the expression of MMP and TIMP as well as iNOS and COX-2 gene expression via directly inhibiting the activation of NF-κB and phosphorylation of MAPK pathway in THP-1 cells. This suggests RVS have potential to be used as a therapeutic agent for acute myeloid leukemia (AML).
Introduction
Acute myeloid leukemia (AML), the most common type of leukemia in adults, is characterized by an uncontrolled proliferation of immature progenitor cells which results in the rapid increase of abnormal cells in blood as well as the bone marrow1). Although AML is a fairly rare disorder, accounting for around 0.9% of all cancers diagnosed in the USA during 20122), its crude incidence rate keeps increasing in USA (from 3.4 (per 100,000) in 2004 to 5.1 in 2013)3) and South Korea (from 1.87 in 1999 to 2.50 in 2012)4). With its increasing incidence with ages, AML steadily raise medical awareness due to its high relapse and poor prognosis. Specifically, approximately 75% of patients with AML relapse after primary therapy without achieving complete remission5), and AML patients showed the shortest 5-year survival rate of 24% among acute leukemia6). In particular, 97% of older AML patients aged 70–79 years will die in the subsequent five years7). However, the underlying pathophysiology of poor survival and frequent relapse of AML still remains elusive. Although multifaceted therapeutic options including active treatment and supportive care are used to treat AML patients, complete recovery rates seem to be low in a majority of cases, particularly elderly patients8).
Upon approaching the devastating cancer of AML, tumor invasion and metastasis are considered important targets for treating aggressive malignancies. Extracellular matrix (ECM) degradation by matrix metalloproteinases (MMPs) are known to increase tumor invasion and thus leading to cancer progression. Among the MMPs family, MMP2 and MMP9 are major proteolytic enzymes in the development of AML due to their increased concentrations in malignant tumor. In particular, MMP2, a significant indicator in response to acute myeloid blast development, is closely associated with tumor invasiveness and relapse9). Tissue inhibitors of metalloproteinases (TIMPs) are important intrinsic factors that regulate MMPs activity in tissues, and have various biological functions as well as inhibiting cell invasion, cancer procession, metastasis and angiogenesis10). The progress of AML is also dependent on the regulation of inflammatory mediators, such as inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2)11), transcriptional activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interaction of the mitogen-activated protein kinases (MAPK) with transcriptional factors12). Therefore, targeting these factors can be promising as a therapeutic approach against hematological cancers.
Rhus verniciflua Stokes (RVS) has long been used for food supplement and traditional herbal medicine in Korea, China, and Japan. Previous researches reported that the RVS has anticancer activity and efficacy on arthritis, etc13, 14). A recent study indicated that the water extract of RVS inhibited the cellular growth of human chronic myelogenous leukemia K562 cells by inducing apoptosis and regulating the NF-κB and MAPK pathways15). However, to date, there is no study investigating the pharmacological effects of RVS against AML cells.
Therefore, the present study aimed to investigate whether RVS extract reverses the levels of MMPs, TIMP, iNOS, and COX-2 expression in THP-1 cells and how it is involved in NF-κB and MAPK signaling pathway.
Materials and methods
1. Rhus verniciflua Stokes (RVS) extract
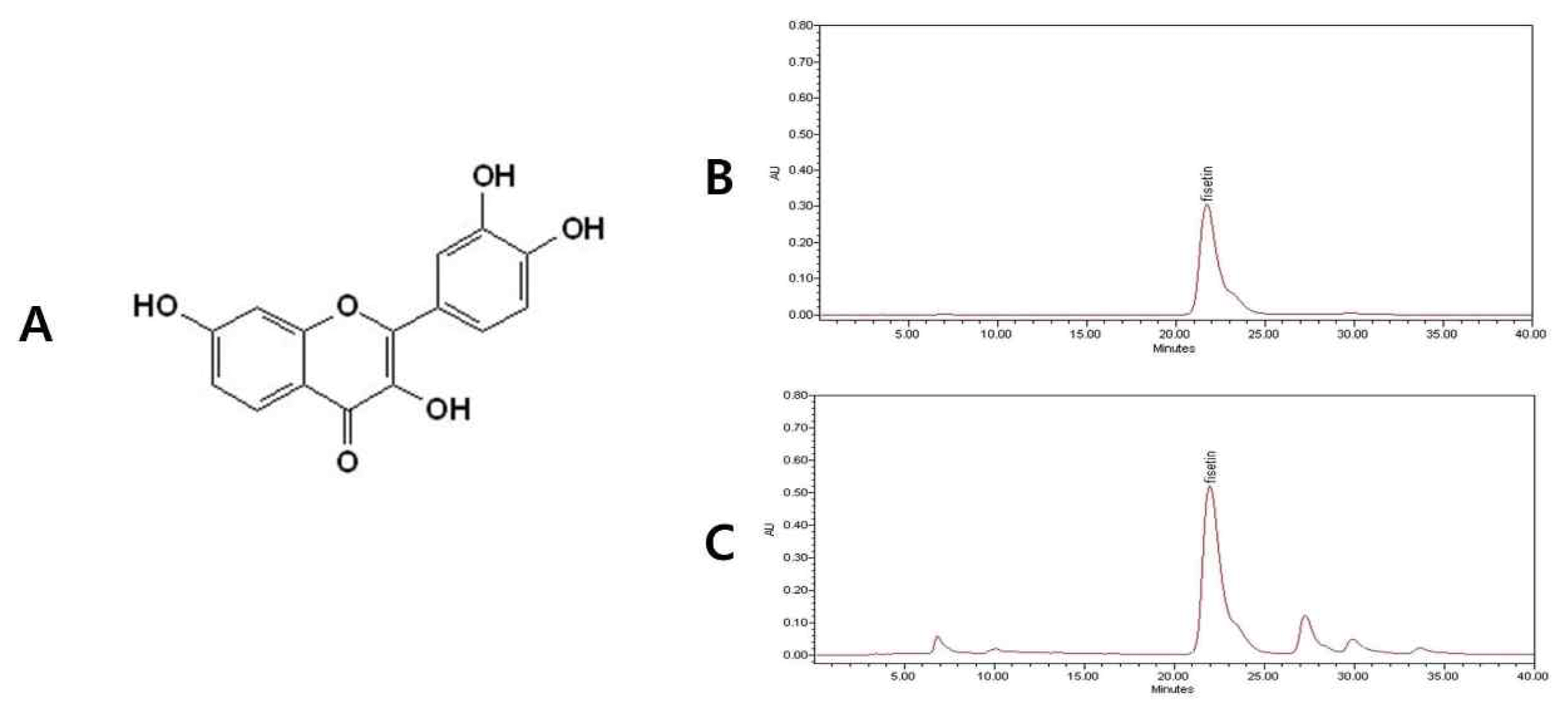
The bark of RVS (produced in Yeosu, Korea) was obtained from Kyung Hee Parm (Wonju, Korea). The herb sample (200 g) was roasted at 180°C for 1 h to remove urushiol that can cause an allergic response (data not shown here) and then boiled twice with 2 liters of distilled water for 3 h, each. The supernatant was collected, evaporated and freeze-dried, yielding 8.6 g of extracted powder (yield, 4.3%). The high-performance liquid chromatography (HPLC) was done to confirm fisetin, an important ingredient of it (Figure 1).
2. Cell culture
THP-1 (Human monocytic leukemia) cells were purchased from the Korean Cell Line Bank. RPMI 1640 (Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum and antibiotics was used as culture medium for this study. Cells were incubated in a humidified atmosphere of 5% CO2 at 37°C in 95% air. THP-1 cells were differentiated into macrophages with 100 nM of phorbol myristate acetate (PMA, Sigma-Aldrich Co., St, Louis, MO, USA) treatment for 72 h. After differentiation, floating cells were discarded, and the attached cells were washed 3 times with culture medium and then incubated.
3. Cell viability
Cell proliferation was assayed with CellTiter 96 Aqueous One Solution (Promega, Madison, WI, USA). Cells were seeded at a concentration of 2 × cells/well in 96-well plates and incubated for 72 h with PMA treatment. The supernatants were removed, and the plated cells were washed with cell culture medium 3 times. And then the cells were incubated in RPMI 1640 medium with different concentrations of RVS for 24, 48 and 72 h, respectively. Cell viability was measured by an assay method using PMS/MTS solution. The absorbance was measured at 490 nm and the background was subtracted at 650 nm.
4. Treatment with RVS extract
THP-1 cells were differentiated with PMA treatment for 72 h. After differentiation, floating cells were discarded, and the attached cells were washed with cell culture medium. THP-1 cells were incubated with RVS (0, 10, 25 and 50 μg/ml) for 24 h in medium containing no serum in it. After incubation with RVS samples, total RNA and protein from the RVS treated THP-1 cells were isolated for analysis.
5. RNA isolation and real-time PCR procedures
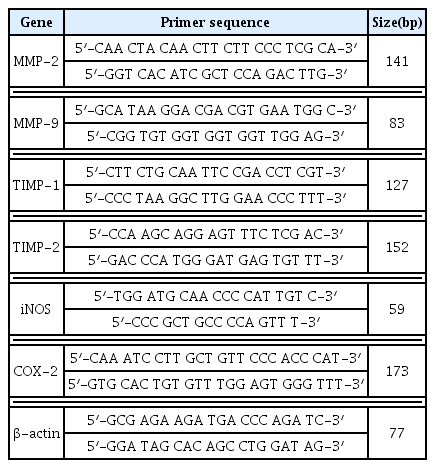
Total RNA was separated from the sample treated cells using the Trizol solution according to the manufacturer’s guidance (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized using 1 μg of total RNA and it was transcribed to cDNA using a reverse transcription system with random hexamers (Promega, Madison, WI, USA) following the manufacturer’s guidance. Primer sequences for this study were as follows (Table 1).
Real-time PCR reaction was performed with StepOneplus real-time PCR systems using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA, USA). RT-PCR was performed with 1 μl cDNA in 20 μl reaction mixtures. The reaction mixture consists of 10 μl Power SYBR Green PCR Master Mix, 2 μl primers, and 7 μl PCR-grade water. The denaturation reaction was performed at 95°C for 10 min, and then followed by 40 cycles of 95°C for 15 sec, and 60°C for 1 min. The crossing point of target genes with β-actin was calculated using the formula 2−(target gene–β actin), and the relative amounts of the target genes were quantified accordingly.
6. Western blot analysis
Cells were harvested and washed with ice-cold PBS then subjected to lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin] containing 1 mM PMSF (Cell Signaling Technology Inc., Boston, MA, USA) to obtain protein lysate. The protein concentration was calculated by a BCA protein assay following the manufacturer’s guidance. Thirty micrograms of protein samples were loaded onto 12% SDS-PAGE for protein resolution and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk for 1 h at room temperature. And the membrane was incubated overnight with primary antibodies against iNOS (Chemicon), COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), NF-κB p65, Akt, C-Jun N-terminal kinases (JNK), extracellular signal-regulated kinase (ERK), p38 MAPK (Cell Signaling Technology), and β-actin (Sigma-Aldrich Co.). These primary antibodies were diluted 1:1,000 with Tris-buffered saline containing 0.05% Tween 20 (TBS-T). After hybridization with the primary antibodies, the membrane was washed with TBS-T for 1 h, and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies diluted 1:2,500 in TBS-T. The membranes were washed with TBS-T for another 1 hour and reactions were developed with Amersham ECL Prime reagent (GE Healthcare Life Sciences, Little Chalfont, UK). Obtained protein band densities were quantified for analysis using ImageJ software (NIH, Bethesda, MD, USA).
7. Statistical analysis
Values are represented as the means ± SD. Data comparison was done by one-way ANOVA (analysis of variance) and Tukey test. The GraphPad Prism 5 software (GraphPad Software Inc., San Diego, USA) was used for statistical analysis. P-values, *p<0.05 and **p<0.01, were accepted as statistically significant.
Results
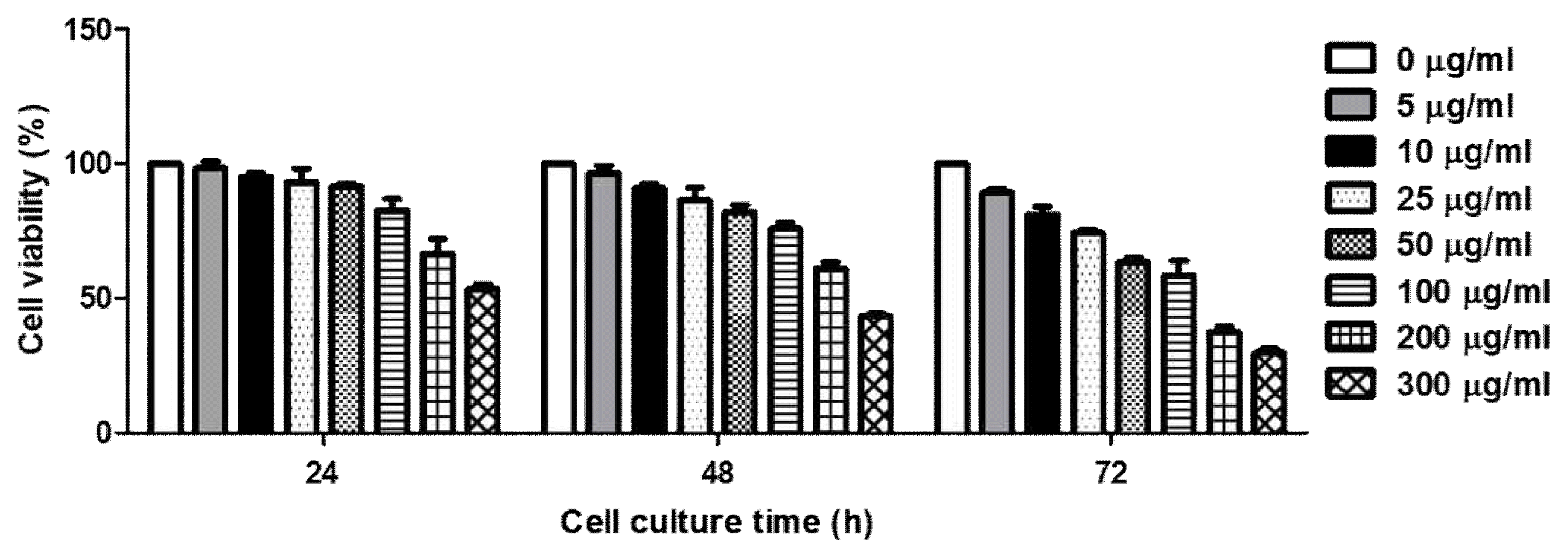
1. RVS extract inhibits cell proliferation
THP-1 cells were exposed to RVS for 24, 48, 72 h each. Cell viability was measured by MTT assay method using MTS/PMS solution. RVS suppressed THP-1 cell proliferation in a dose-and time-dependent manner. When the treatment concentrations of RVS are 50 ug/ml or less for 24 h, there was no cytotoxicity observed (Figure 2).
2. RVS inhibited MMP and TIMP mRNA expressions
THP-1 cells were exposed to RVS (10, 25 and 50 μg/ml) for 24 h. The expressions of MMPs and TIMPs mRNA were measured by real-time PCR. MMP-2 and MMP-9 mRNA levels tended to decrease as RVS concentration increased (Figure 3). TIMP-1 and TIMP-2 also tended to decrease depending on the treatment concentration and showed statistically significant difference at 50 μg/ml (Figure 3).
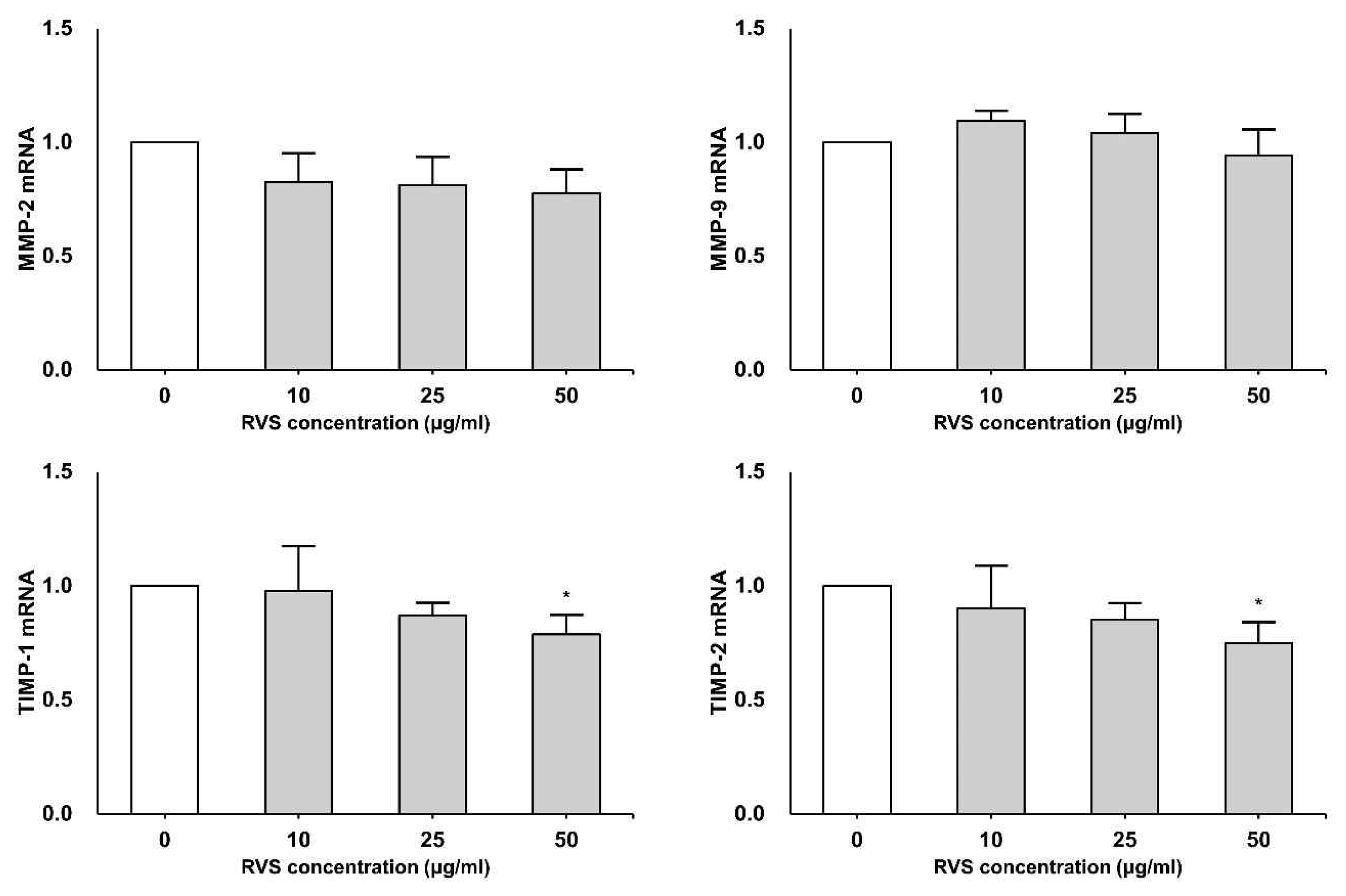
Expression of MMP and TIMP mRNAs in THP-1 cells
Expression of mRNA was measured using real-time PCR. The relationships of MMPs and TIMPs with β-actin were calculated using the formula, 2−(target gene − β-actin) and the relative quantities of transcripts were analyzed. The data are shown as means ± SD of three independent experiments. (*P<0.05, **P<0.01; compared to control.)
3. RVS inhibited the expression of iNOS and COX-2 mRNA genes and proteins
THP-1 cells were exposed to RVS (10, 25 and 50 μg/ml) for 24 h. The expressions of mRNA and protein were measured by real-time PCR and western blot. RVS suppressed the expression of iNOS and COX-2 mRNA and protein compared to the control (Figure 4).
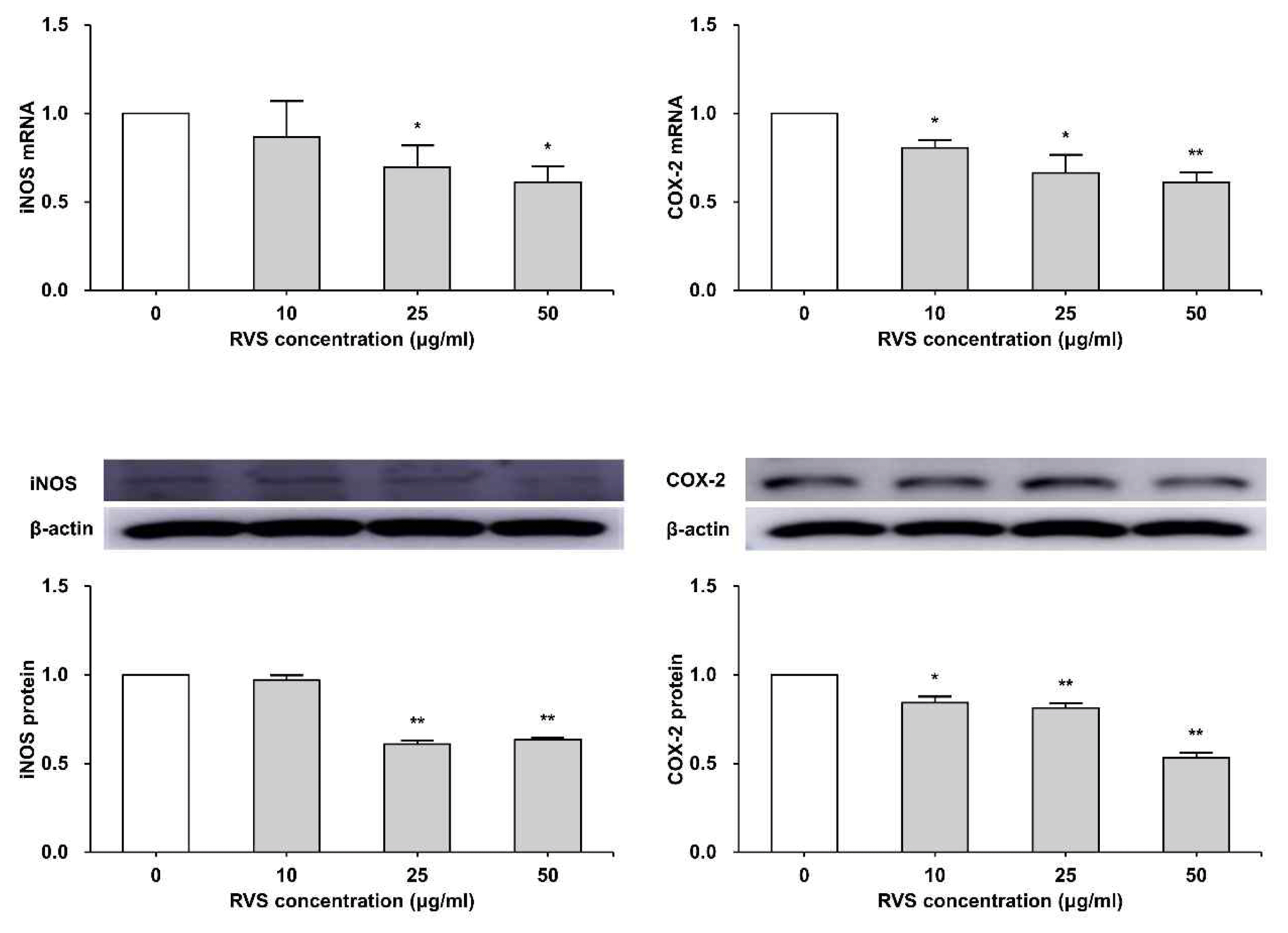
Expression of iNOS and COX-2 mRNAs and protein in THP-1 cells
Expression of mRNA was measured using real-time PCR. (A) The relationships of iNOS and COX-2 with β-actin were determined using the formula, 2−(target gene − β-actin) and the relative quantities of transcripts were measured. Expression of protein was examined by western blot. (B) Densitometric analysis is presented as the relative rations of iNOS and COX-2 to β-actin. The data are shown as means ± SD of three independent samples. (*P<0.05, **P<0.01 compared to control.)
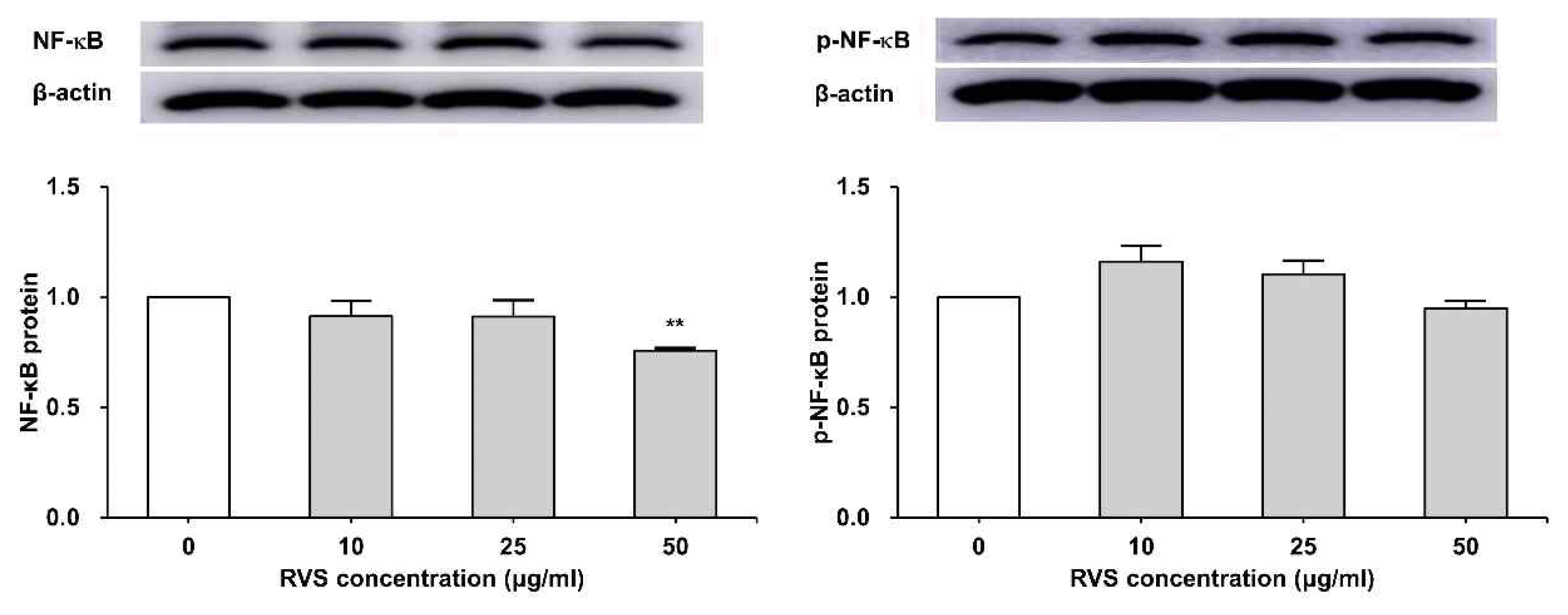
4. RVS inhibited NF-κB p65 protein expression
THP-1 cells were exposed to RVS (10, 25, and 50 μg/ml) for 24 h. The expression of NF-κB p65 protein was determined by western blot analysis. NF-κB p65 total protein was decreased depending on the concentration of RVS. The phosphorylation of NF-κB p65 protein level was increased at a low concentration but it showed decreasing tendency as RVS concentration increased (Figure 5).
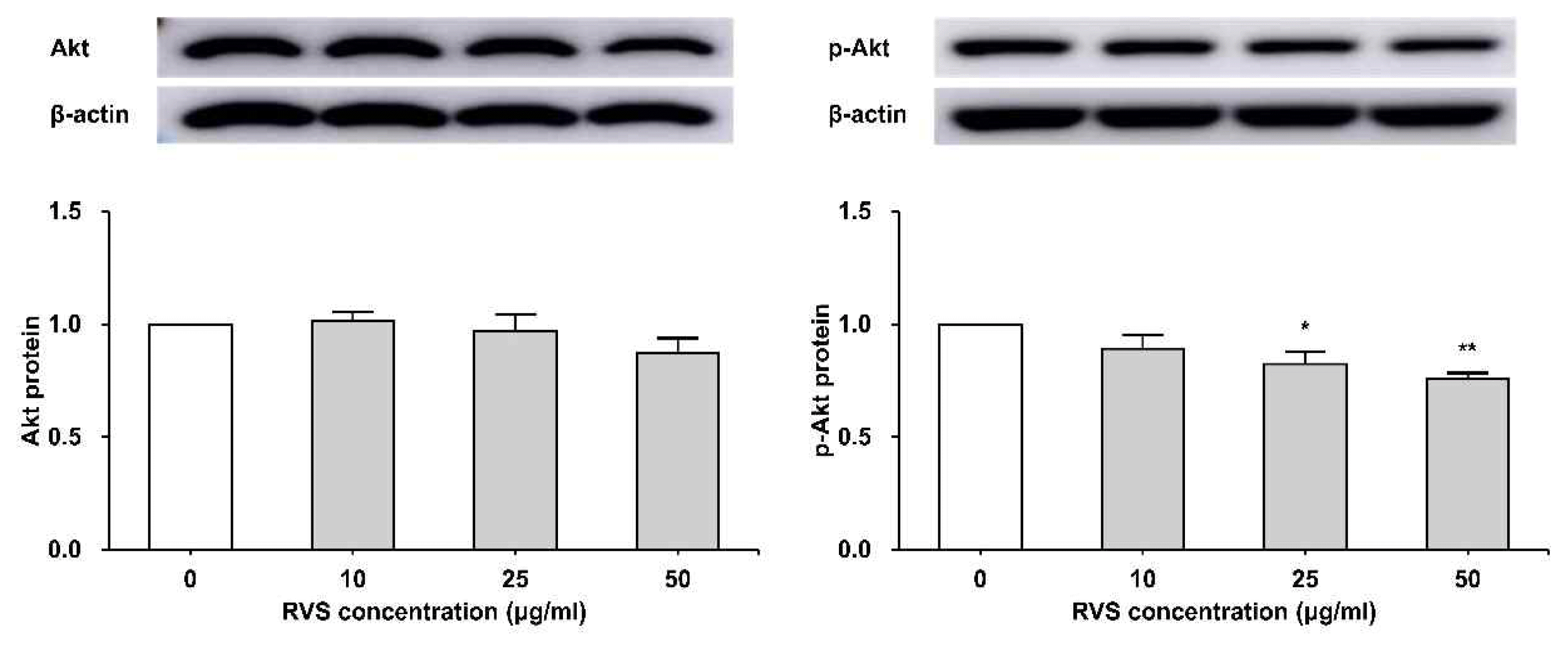
5. RVS inhibited phosphorylation of Akt protein
THP-1 cells were exposed to RVS (10, 25, and 50 μg/ml) for 24 h. The expression of Akt protein was examined by western blot analysis. The expression of total Akt protein tended to decrease as RVS concentration increased, but statistically significant. The phosphorylation of Akt protein showed a statistically significant decrease compared to the control as RVS concentration increased (Figure 6).
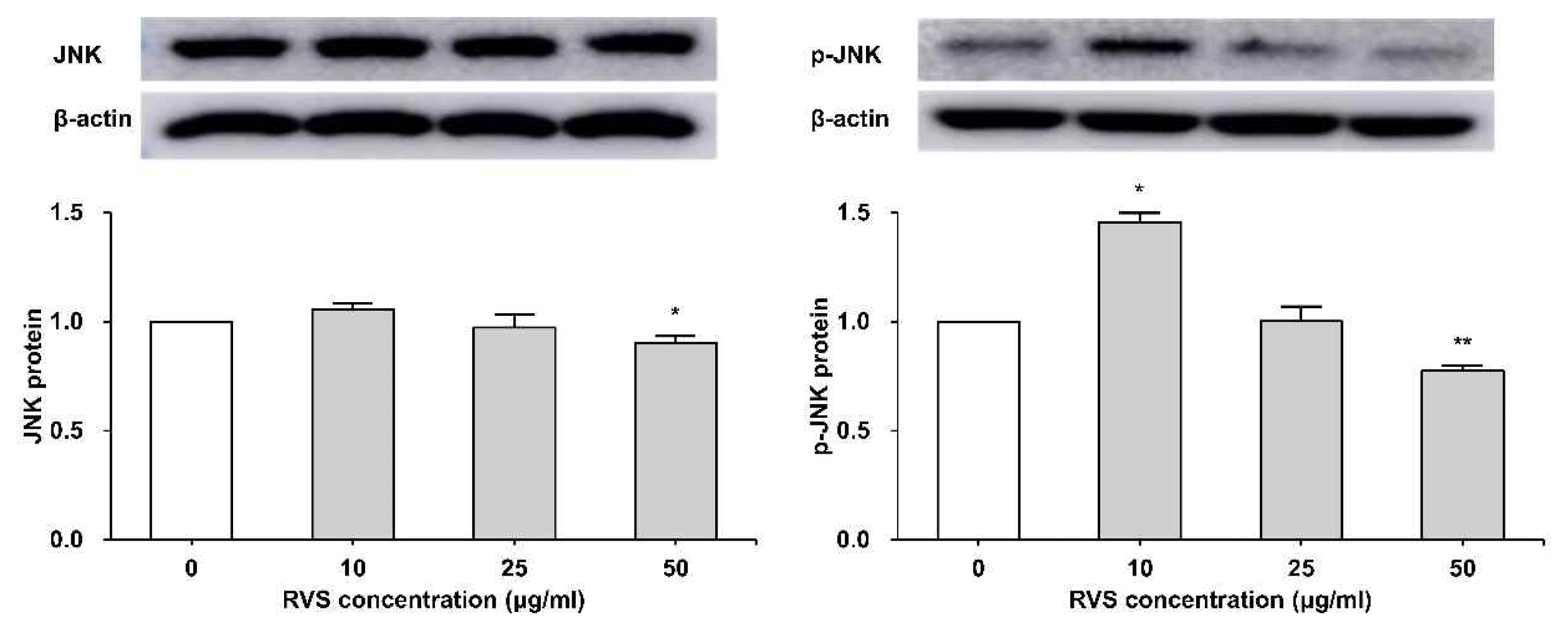
6. RVS inhibited phosphorylation of JNK protein
THP-1 cells were exposed to RVS (10, 25, and 50 μg/ml) for 24 h. The expression of JNK protein was examined by western blot analysis. Total JNK protein was slightly increased at 10 μg/ml, but it was decreased as RVS concentration increased. Moreover, it was confirmed that the expression of JNK protein showed a statistically significant decrease at 50 μg/ml (p<0.05). The phosphorylated JNK showed a statistically significant increase at 10 μg/ml but p-JNK decreased as RVS concentration increased, and a statistically significant decrease was shown at 50 μg/ml (Figure 7).
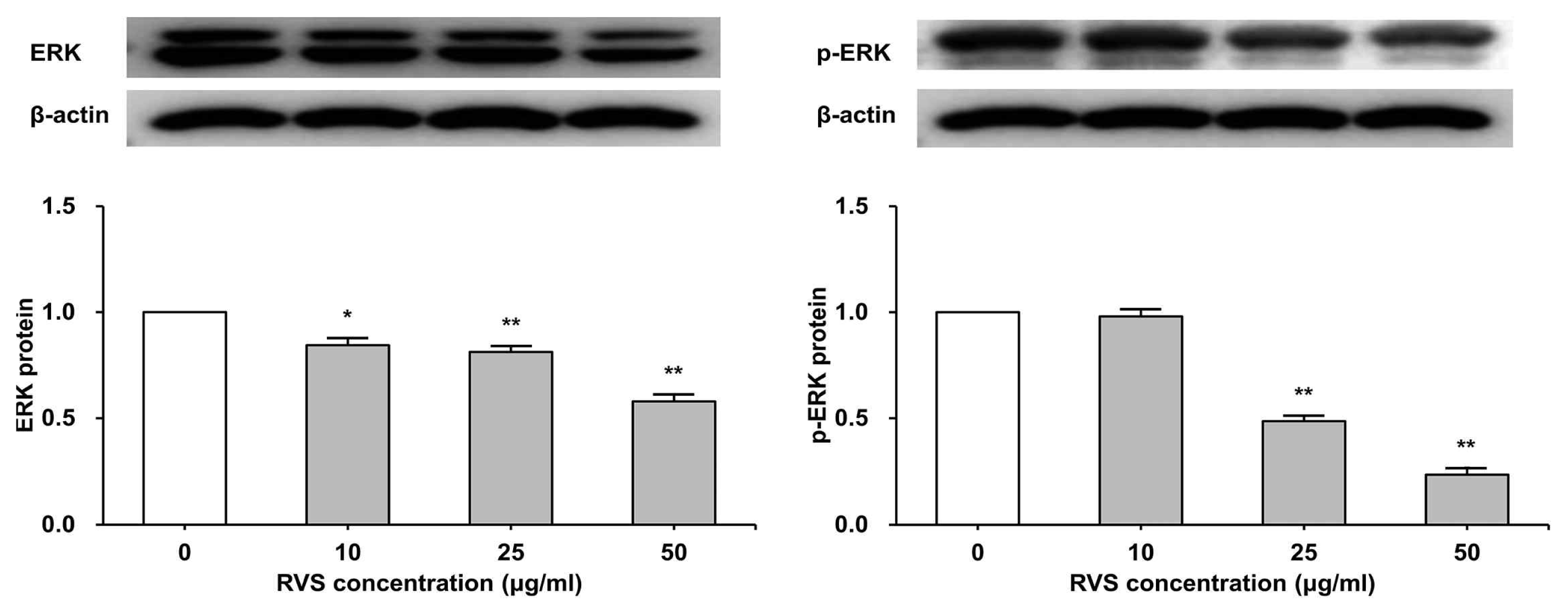
7. RVS inhibited phosphorylation of ERK protein
THP-1 cells were exposed to RVS (10, 25, and 50 μg/ml) for 24 h. The expression of ERK protein was examined by western blot. The expression of total ERK decreased significantly as RVS concentration increased, and the phosphorylated ERK protein showed statistically significant decrease compared to the control at 25 and 50 μg/ml (Figure 8).
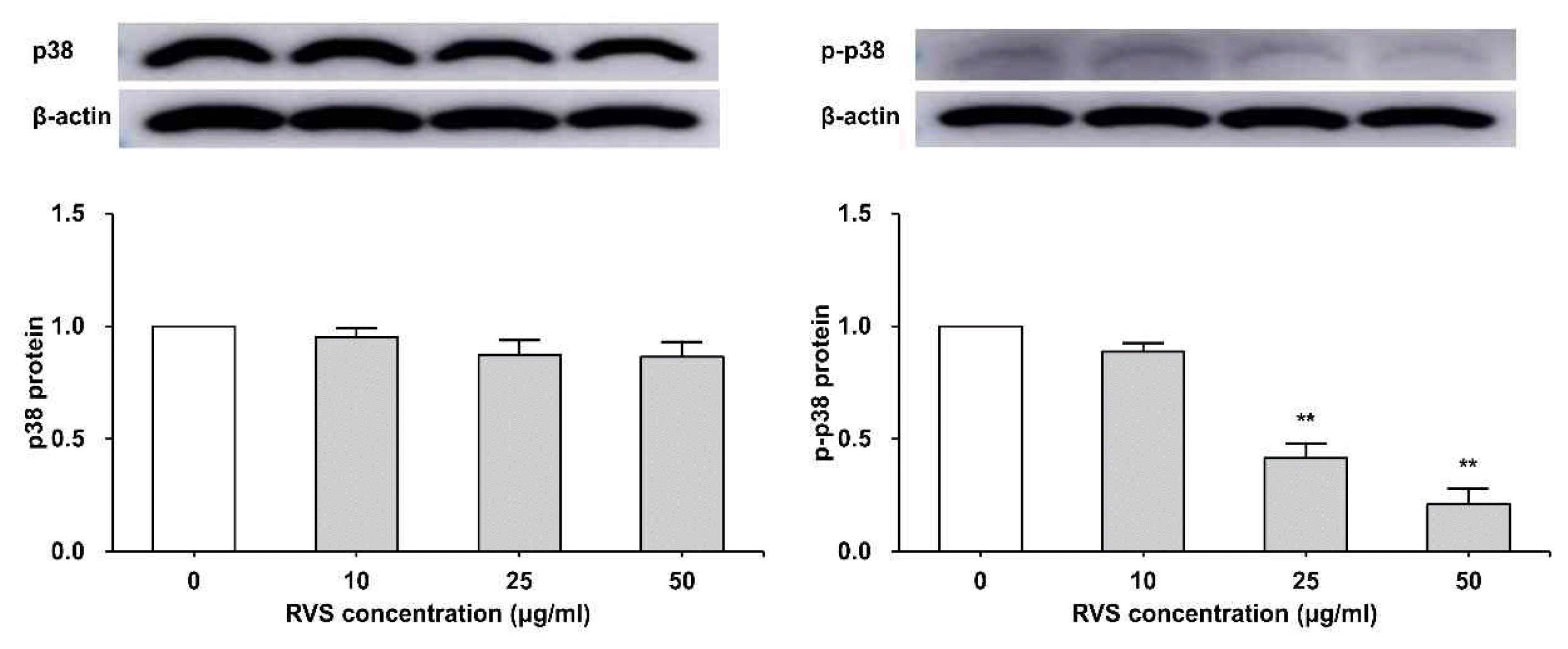
8. RVS inhibited phosphorylation of p38 protein
THP-1 cells were exposed to RVS (10, 25, and 50 μg/ml) for 24 h. The expression of p38 protein was examined by western blot. The expression of total p38 protein tended to decrease as RVS concentration increased, and the phosphorylated p38 protein showed a statistically significant decrease compared to the control at 25 and 50 μg/ml (Figure 9).
Discussion
Despite years of innumerable researches in the field of biomedicine to understand the mechanisms of tumorigenesis and pathogenesis of cancers, conventional treatments including surgery, radiation therapy, and chemotherapy are still the main options, though each has its own limitations. Recently, there are growing global interests in natural medicines derived from natural resources owing to less adverse reaction and better tolerance as compared to conventional synthetic medicines16). Emerging evidences have revealed that herbal remedies can inhibit proliferation, adhesion and migration of cancer cells while inducing apoptosis of them. In addition to this direct anticancer effect, several herbal remedies exhibited suppressing effect on angiogenesis and thus reducing tumor growth17).
Acute myeloid leukemia (AML), the most common type of leukemia in adults, results in the rapid increase of abnormal cells in blood as well as the bone marrow. According to research of AML, tumor invasion and metastasis are considered important targets for treating aggressive malignancies. In the treatment of AML, there is a need for research on anti-cancer effects of various herbal medicines.
Many herbs contain substances such as terpenes, phenols and polyphenols with anti-tumor activity. Plant polyphenols can be divided into flavonoids and nonflavonoids16). Plant polyphenols, in many researches, showed much properties of preventing cancer development. Which includes antioxidant activity, radical scavenging activity, carcinogen inactivation, inhibition of cell proliferation and angiogenesis, cell cycle arrest, pro-apoptotic activity, modulation of tumor suppression genes, etc18). Some studies have shown that various herbs such as Ginseng root, Allium sativum and Typhonium flagelliforme are effective for treatment of leukemia19).
Rhus verniciflua Stokes (RVS) is used as traditional herbal medicine as well as a food ingredient in Korea. RVS also contains various flavonoids having anti-inflammatory and anti-tumor activity, and many related studies have been reported. RVS extract has been used for cancer treatment in traditional medicine in Korea and is beneficial to some patients with advanced cancer though it is not a single compound but a herbal extract showing low cytotoxic in vitro experiments20).
In this study, cell viability assay was done in the THP-1 cells exposed to RVS with different concentrations and time points. The cell count number was barely decreased at a dose of 50 μg/ml or lower for 24 h treatment. The maximum dose of RVS was set at 50 μg/ml and treated for 24 h to evaluate possible mechanisms of its action. It is to focus on anti-inflammatory effect of RVS with least cytotoxic concentration and exposure time.
This study was done to have an insight on the effect of RVS on THP-1 cells which is derived from human monocytic leukemia. In the present study, the expressions of MMP-2 and MMP-9 mRNA tended to decrease as RVS concentration was increased. These results suggest that RVS inhibits MMP-2 and MMP-9 transcription in THP-1 cells thus suppressing tumor cell migration and invasion. MMP family is involved in angiogenesis of tumor by MMP-2, MMP-9, and MMP-1421). Similar results were recognized that Orostachys japonicas suppressed MMP-2 and MMP-9 transcription in LnCaP (human prostate cancer) cells and THP-1 cells22). TIMP is an important regulatory factor and is involved in cellular activity as well as in the formation of cellular matrix. This study has shown that RVS reduced TIMP-1 and TIMP-2 mRNA expression. The result indicates that RVS inhibits proliferation and invasion of cancer cells through inhibiting TIMP-1 and TIMP-2 in THP-1 cells. TIMP-1 mRNA was well expressed in non-small cell lung cancer of a patients23). TIMP-2 was expressed in human breast cancer24) and predicts prognosis of colorectal cancer via regulating MMP-924).
Taken together, inhibition of MMP and TIMP mRNA expression has been shown to mediate growth factor stimulated proliferation, angiogenesis, and invasion and metastasis of tumors. MMP-2 and MMP-9 mRNAs show high levels in breast cancer and the survival rate is inversely correlated. Measurement of the MMP-2 and MMP-9 mRNA possibly enables to anticipate the biological malignancy of cancer24).
In the present study, the production of iNOS and COX-2 genes and proteins were reduced by RVS treatment. In a similar study to this experiment, Rhododendron album Blume also showed inhibition of iNOS and COX-2 expression in LPS-treated RAW264.7 cells which is supposedly related with downregulation of NF-κB signaling25). These results suggest that RVS controls iNOS and COX-2 gene expression with similar pathways. While the expression of iNOS and COX-2 induces cell proliferation and inhibits apoptosis, they have the advantages of contributing to wound healing and protection of the gastric mucosa, they also can increase the possibility of malignancy development. Although the exact mechanism is unknown, the expression of iNOS and COX-2 mRNA in cancer tissues is presumably closely related26).
NF-κB stimulates tumor cell proliferation, prevents apoptosis, regulates tumor angiogenesis and promotes tumor metastasis27). As a major transcription factor, NF-κB regulates gene expressions and is activated through distinct signaling components28), and it plays an important role in signaling pathway involved in pathogenesis and treatment of cancer29). In this study, RVS tended to suppress the activation of the NF-κB p65 phosphorylation as RVS concentration was increased. These results suggest that RVS controlled the phosphorylation of NF-κB signaling pathway by mediating signal transduction. Cordyceps militaris extract inhibits the NF-κB and induces apoptosis via activation of MKK7-JNK signaling in human renal cell carcinoma TK-10 cells30). Shikonin decreased MMP-2 and MMP-9 levels via inhibiting Akt and NF-κB activation31). Phosphorylation of inhibitory κBα (IκBα) activates NF-κB via activation of several MAPKs which includes c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK)32,33).
In the present study, the phosphorylation of Akt by RVS treatment is decreased, and the phosphorylation of JNK is increased at a very low concentration but decreased at higher concentrations. Akt activation protects against apoptosis, and JNK increases apoptosis through regulation of bax/bcl-2/caspase activation34). In a previous study, RVS suppressed inflammation by down-regulation of c-Jun N-terminal kinase (JNK) pathway in lipopolysaccharide (LPS) induced RAW264.7 macrophages.
However, ethanol-extracted RVS activated JNK. The authors asserted that this discrepancy may be caused by different experimental conditions, such as cell type, extraction method and the concentration of RVS35). In this study, the decrease of the expression of p-JNK as RVS concentration increased shows the possibility that RVS treatment suppressed cell proliferation when RVS concentration is high enough.
The MAPK is important pathway in signal transduction, which includes JNK, ERK, and p38 MAPK36). In the present study, RVS showed suppressing activities on the phosphorylation of ERK and p38 MAPK. This finding suggests that RVS can modulate the expressions of MMP, TIMP, iNOS, and COX-2 in THP-1 cells through MAPKs signaling, including Akt. Similar results were observed in a study on the effect of Orostachys japonicus which inhibited LPS-induced NF-κB activation and IκBα degradation and suppressed LPS-induced JNK, p38 MAPK, and ERK phosphorylation37). They seem to share similar properties in the reaction. And this finding provides an idea of combining herbs for better effect. There is an example of combining herbs for treatment. An herbal formula, APR, containing Angelica gigas Nakai, Panax ginseng and Rhus verniciflua Stokes, inhibited AKT, ERK, p38 and NF-κB. APR also suppressed iNOS and Cox-2 mRNA induced by LPS pretreatment in cell culture model38,39).
In conclusion, RVS is considered to suppress the expressions of matrix metalloproteinases, iNOS and COX-2 mRNAs and proteins, which is influenced by inhibiting the activation of NF-kB and phosphorylation of the MAPK (ERK and p38) pathway in THP-1 cells. The results suggest that RVS has anti-inflammatory effect as well as anti-cancer effect on patients with leukemia. Many previous studies had proved that RVS has anti-cancer effect. In this report, it is further confirmed RVS is effective to suppress not only the growth of cancer directly, but also inflammation that can act as a predisposing factor. And there are numerous herbal prescriptions containing many herbs in a formula for various kinds of diseases in traditional Korean medicine. In most of the cases herbal formula exerts stronger effects when effective herbs are combined. There are several researches on herbs that have anti-inflammatory and anti-cancer effects such as Orostachys japonicus and Panax ginseng. It is possible to develop new herbal formulas having better effect on cancers. This result may lead to further studies on the effect of RVS as well as the combination with other known anti-inflammatory herbs.