Anti-inflammatory Effects of Ojeok-san in LPS-induced Inflammatory Rat Model
Article information
Abstract
Objectives
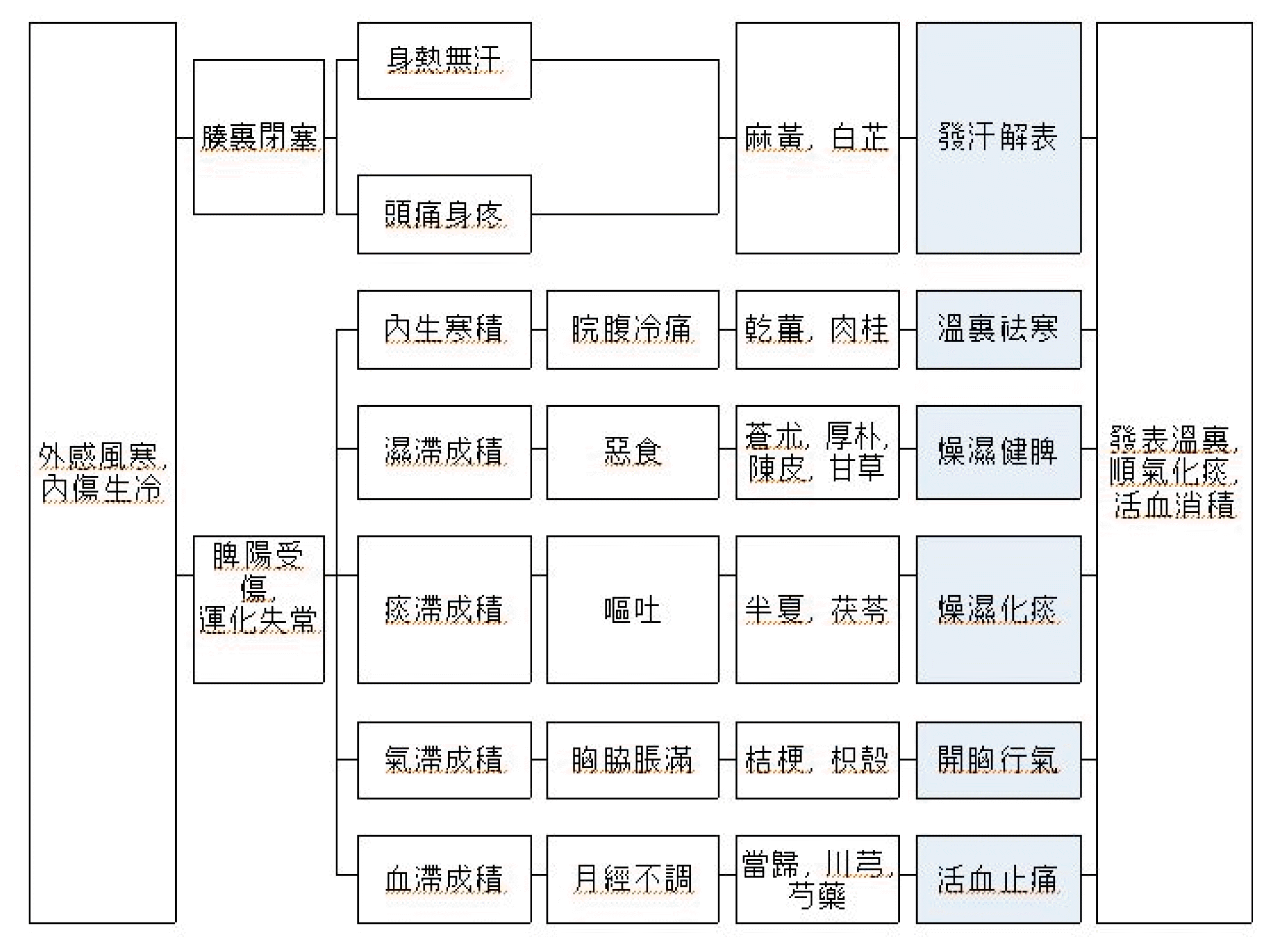
The aim of this study is to investigate the anti-inflammatory effects of Ojeok-san and compare the therapeutic effects according to its formation.
Methods
We evaluated the anti-inflammatory effects of Ojeok-san using lipopolysaccharide (LPS) induced inflammatory animal model. Male SD rats were administered intra-orally with two different formulation types of Ojeok-san according to prescribed dosage. One hour later, to induce inflammatory responses, subsequent intra-peritoneal injection of LPS was conducted. After 5 hours later, serum TNF-α, IL-1β, IL-6 and PGE2 levels were measured by ELISA to assess the alteration of pro-inflammatory markers.
Results
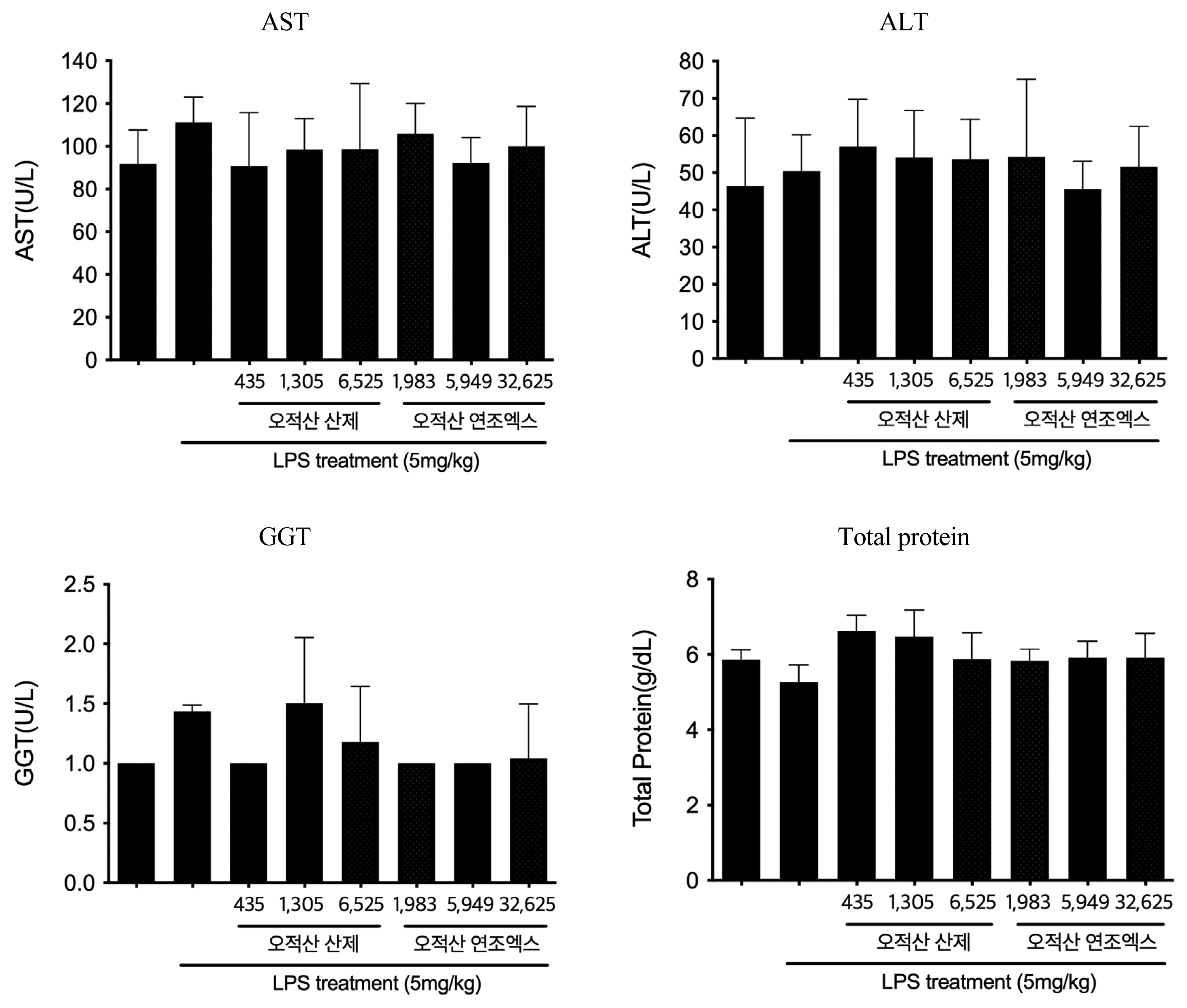
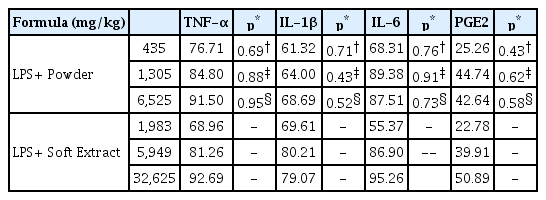
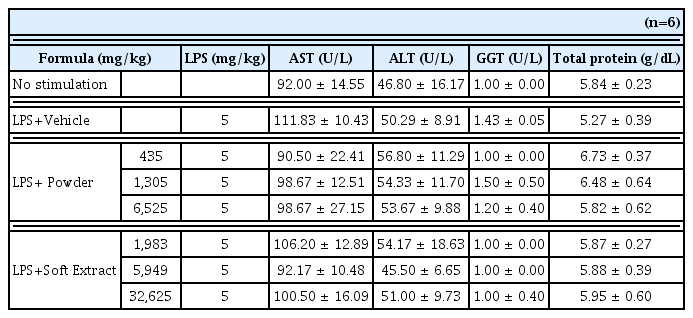
In our experiment, regardless of its formation, administration of Ojeok-san decreased TNF-α, IL-1β, IL-6 and PGE2 level in serum. Furthermore, LPS-induced toxicity of liver and kidney was not detected by Ojeok-san administration.
Conclusions
The anti-inflammatory effect of Ojeok-san was shown in LPS-induced inflammatory model by decreasing pro-inflammatory markers, and there would be no significant difference in therapeutic effect between two formulation types of Ojeok-san.
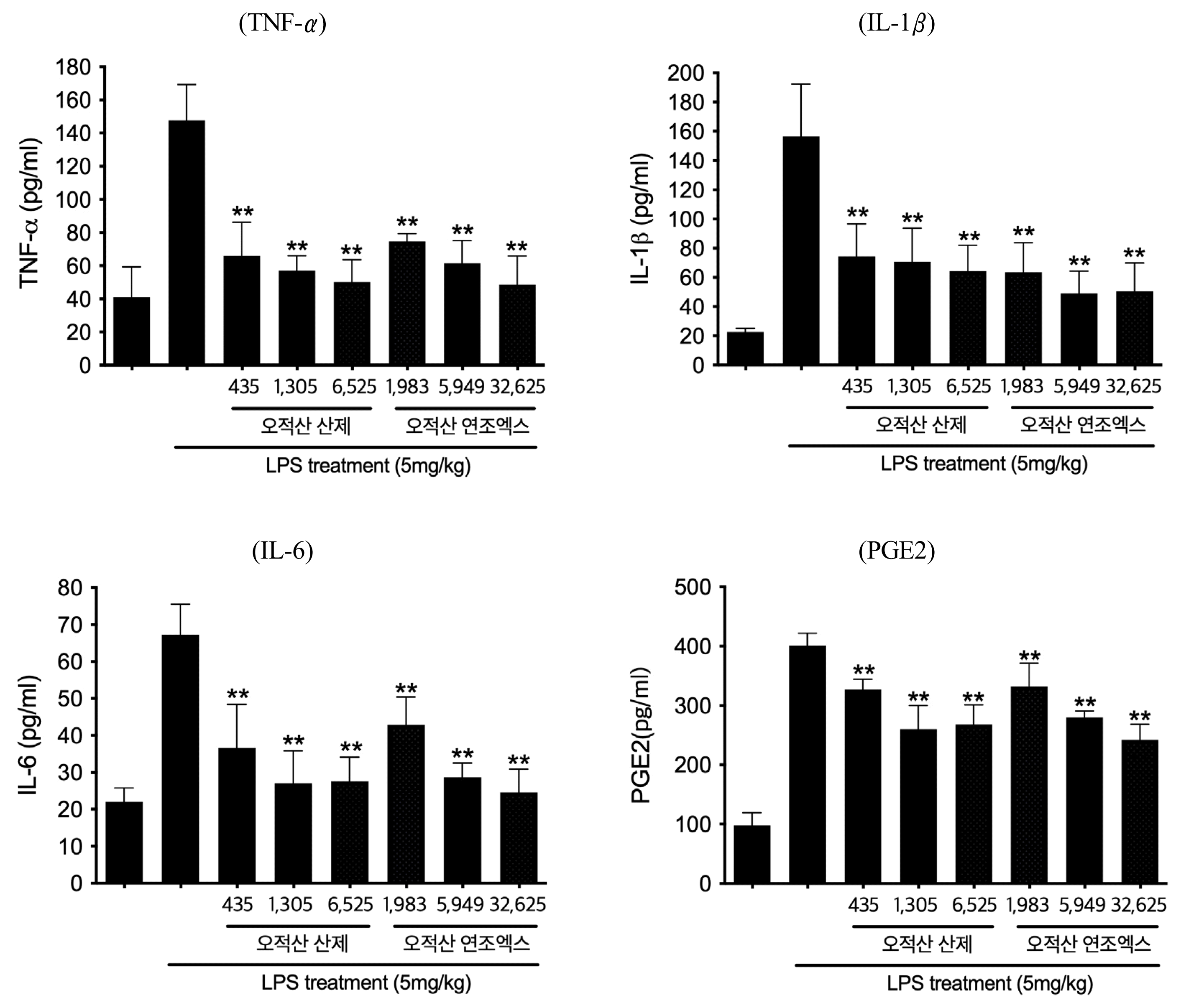
Anti-inflammatory effects between Ojeok-san Extract Powder group and Soft Extract groups in LPS induced inflammatory models (n=5, mean±SD, p: p value of ANOVA test vs. LPS + Vehicle group; *: p<0.05, **: p<0.01)
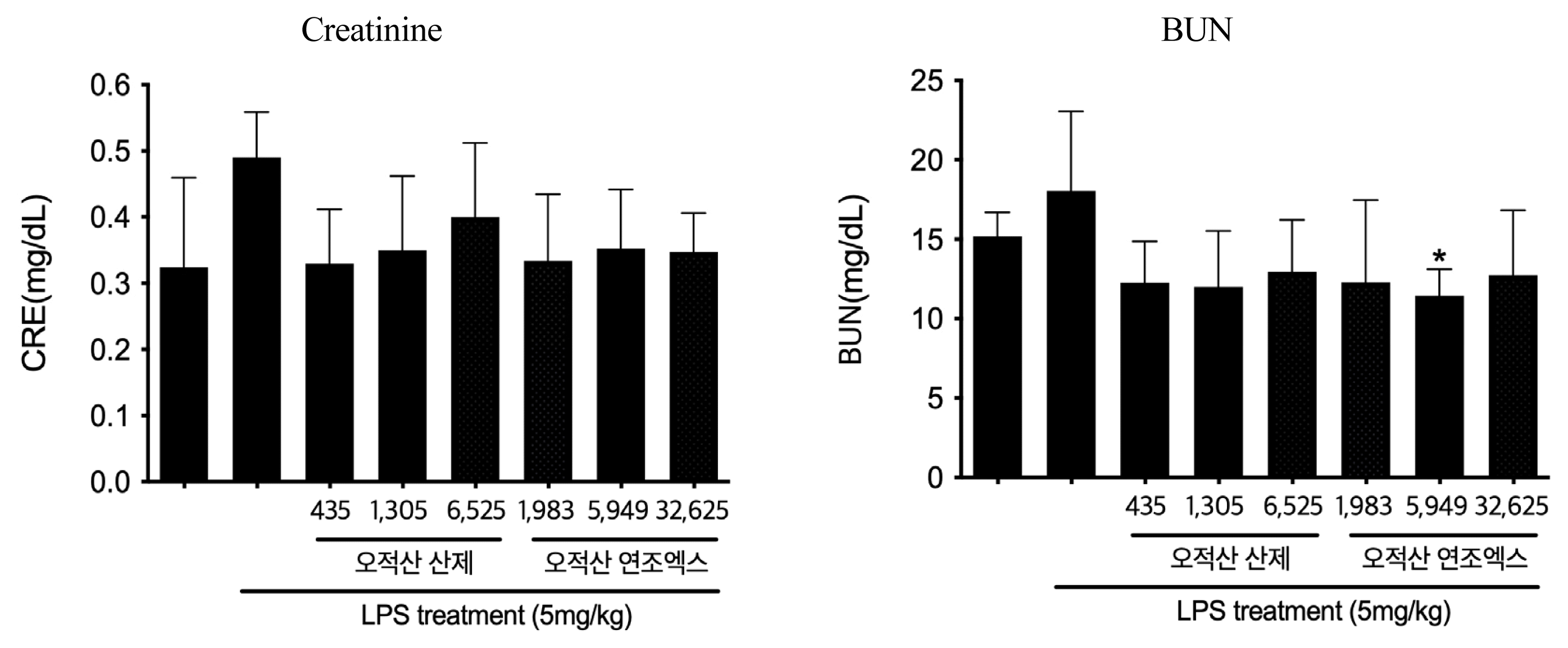
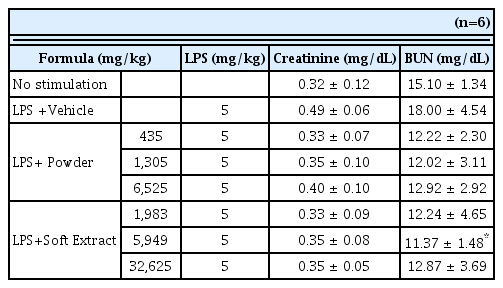
Kidney function test results after the injection of LPS according to the groups
(n=6, mean±SD; p: p value of ANOVA test vs. LPS + Vehicle group ; *: p<0.05)
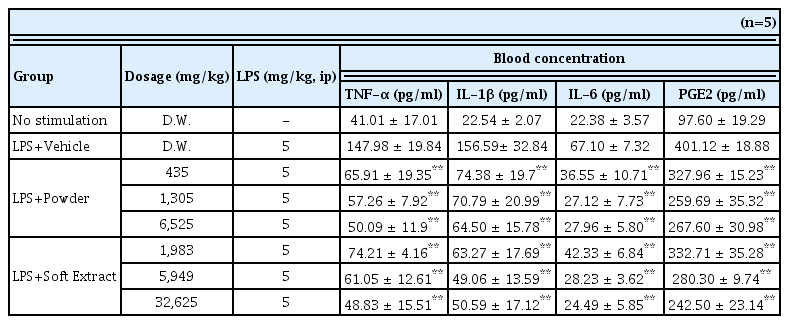
Comparison of anti-inflammatory effects between Ojeok-san Extract Powder group and Soft Extract groups in LPS induced inflammatory models
Acknowledgement
This work was supported by a grant from Pharmacological Standardization Project of Traditional Korean Medicine by Ministry of Health and welfare (MOHW) and JeollaNamdo, Republic of Korea.