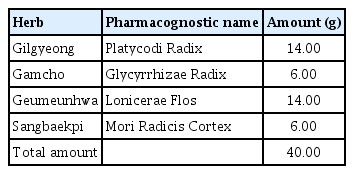
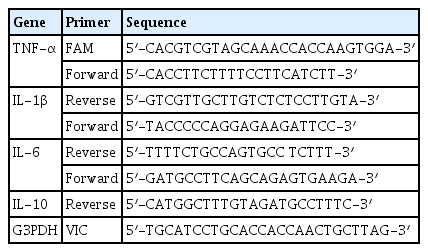
Inhibitory Effects of GGX on Lung Injury of Chronic Obstructive Lung Disease (COPD) Mice Model
Article information
Abstract
Objectives
This study is aimed to evaluate the protective effects of GGX on lung injury of Chronic Obstructive Lung Disease (COPD) mice model.
Materials and Methods
C57BL/6 mice were challenged with lipopolysaccharide (LPS) and cigarette smoke extract (CSE) and then treated with vehicle only (Control group), dexamethasone 3 mg/kg (Dexa group), gam-gil-tang 200 mg/kg (GGT group), GGX 100, 200, and 400 mg/kg (GGX group). After sacrifice, its bronchoalveolar lavage fluid (BALF) or lung tissue was analyzed with cytospin, Enzyme-Linked Immunosorbent Assay (ELISA), real-time polymerase chain reaction (PCR) and hematoxylin & eosin (H&E), and Masson’s trichrome staining.
Results
In the COPD model, GGX significantly inhibited the increase of neutrophils, TNF-α, IL-17A, CXCL-1, MIP2 in BALF and TNF-α, IL-1β, IL-10 mRNA expression in lung tissue. It also decreased the severity of histological lung injury.
Conclusion
This study suggests the usability of GGX for COPD patients by controlling lung tissue injury.
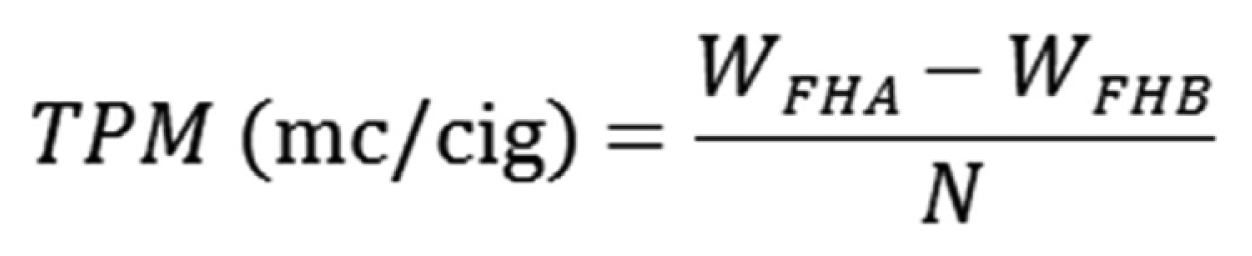
Total particulate matter of cigarette smoke solution.
TPM: total particulate matter, WFHA: Weight of filter holder after smoke, WFHB: Weight of filter holder before smoke, N: Cigarette number of each trap.
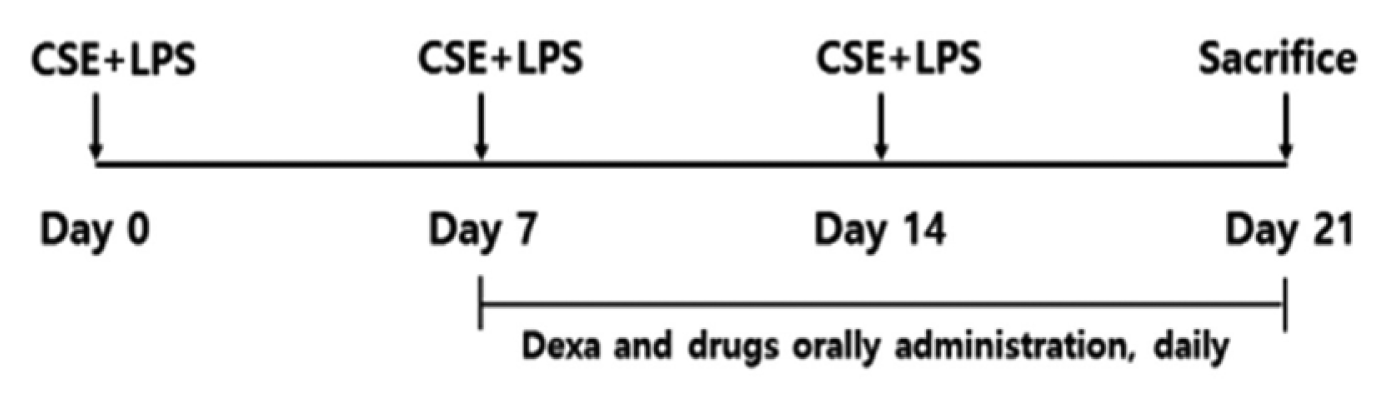
Experimental plan of repeated CSE+LPS exposure. CSE+LPS: Intranasal instillation of LPS 100 μg/μl and cigarette smoke extract 1 mg/ml.
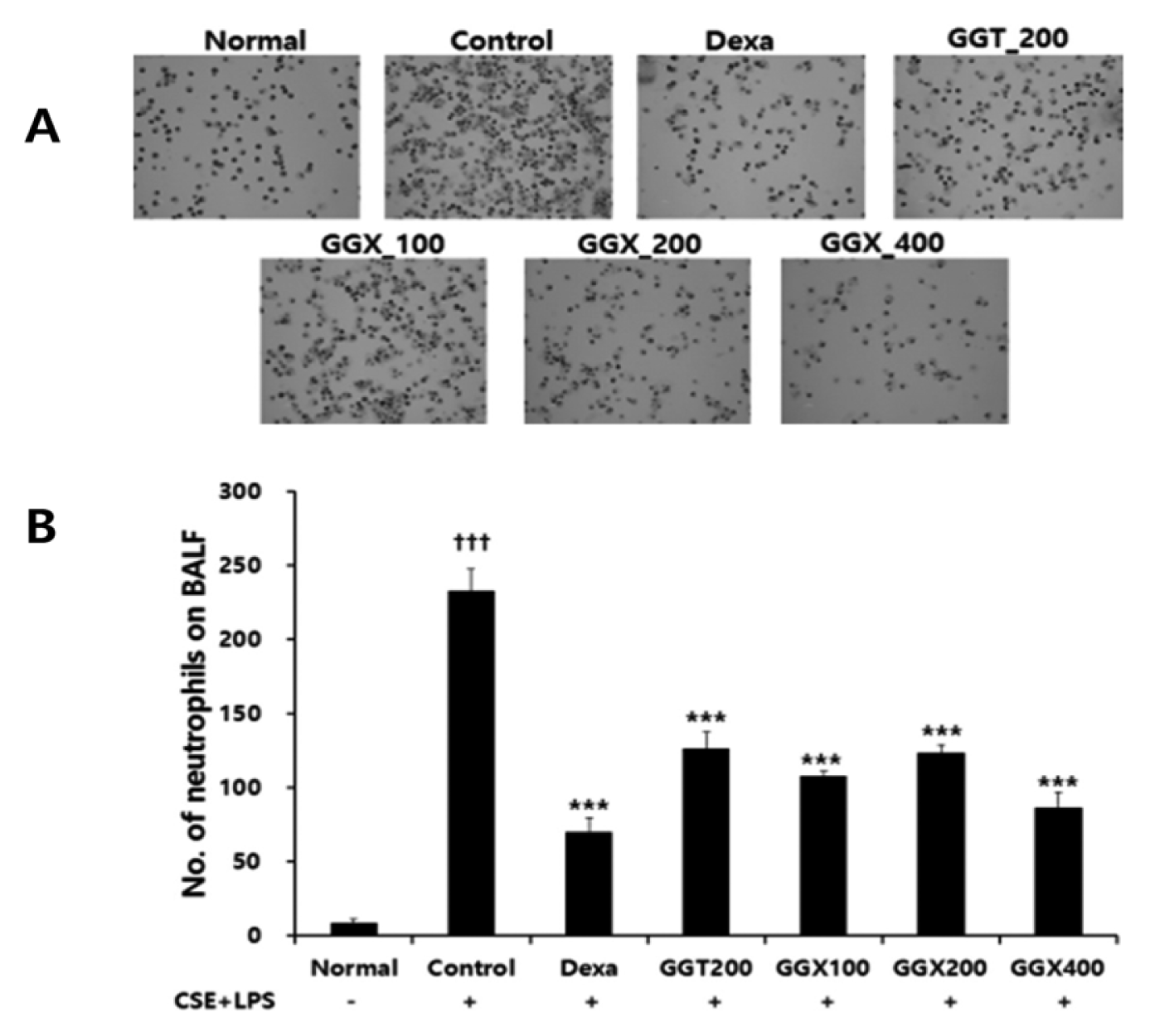
Effect of GGX on cytospin image (A) and neutrophils count (B) in BALF of COPD mice.
Mice were challenged by aspiration of LPS+CSE (Control), and then treated with Dexa (dexamethasone 3 mg/kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=8). All values are presented as mean±SE. †: Significant difference with the non-treated group (††† p<0.001), *: Significant difference with the Control (*** p<0.001).

Effect of GGX on TNF-α production in BALF of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=8). Level of TNF-α was determined with ELISA. All values are presented as mean±SE. †: Significant difference with the non-treated group (††† p<0.001), *: Significant difference with the Control (** p<0.01, *** p<0.001).
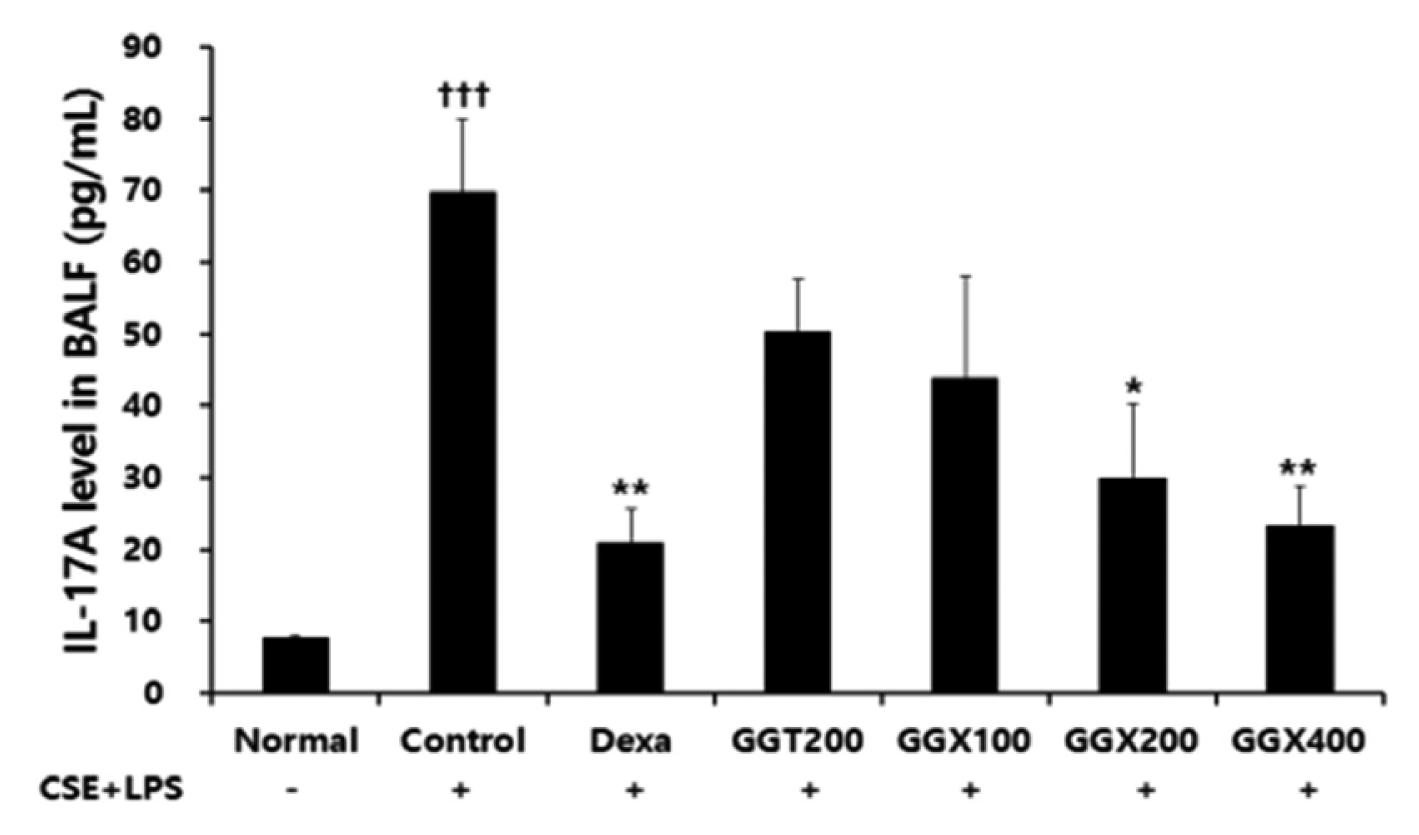
Effect of GGX on IL-17A production of BALF in COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=8). Level of TNF-α was determined with ELISA. All values are presented as mean±SE. †: Significant difference with the non-treated group (††† p<0.001), *: Significant difference with the Control (* p<0.05, ** p<0.01).
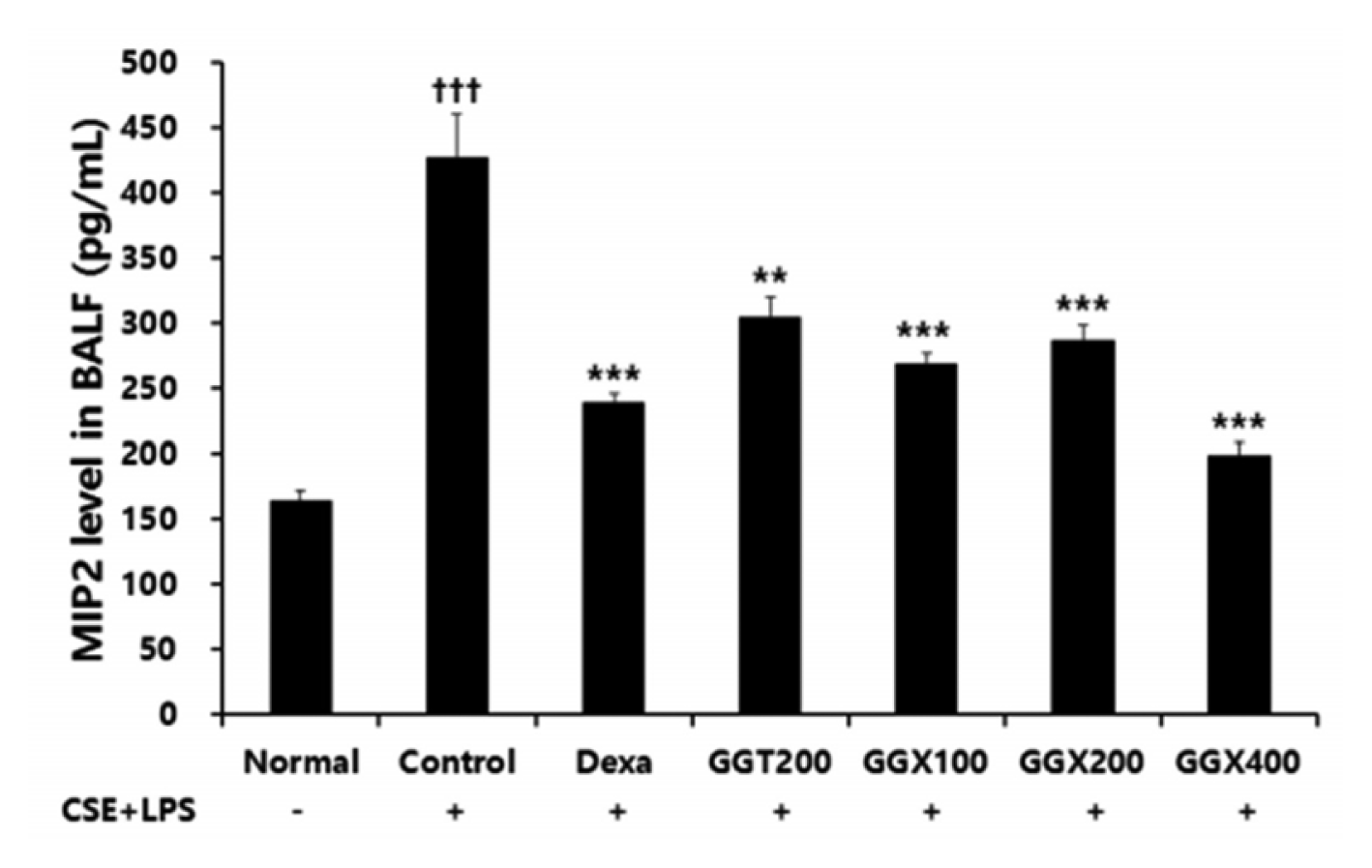
Effect of GGX on MIP2 production in BALF of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=8). Level of TNF-α was determined with ELISA. All values are presented as mean±SE. †: Significant difference with the non-treated group (††† p<0.001), *: Significant difference with the Control (** p<0.01, *** p<0.001).

Effect of GGX on CXCL-1 production in BALF of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=8). Level of TNF-α was determined with ELISA. All values are presented as mean±SE. †: Significant difference with the non-treated group (††† p<0.001), *: Significant difference with the Control (*** p<0.001).
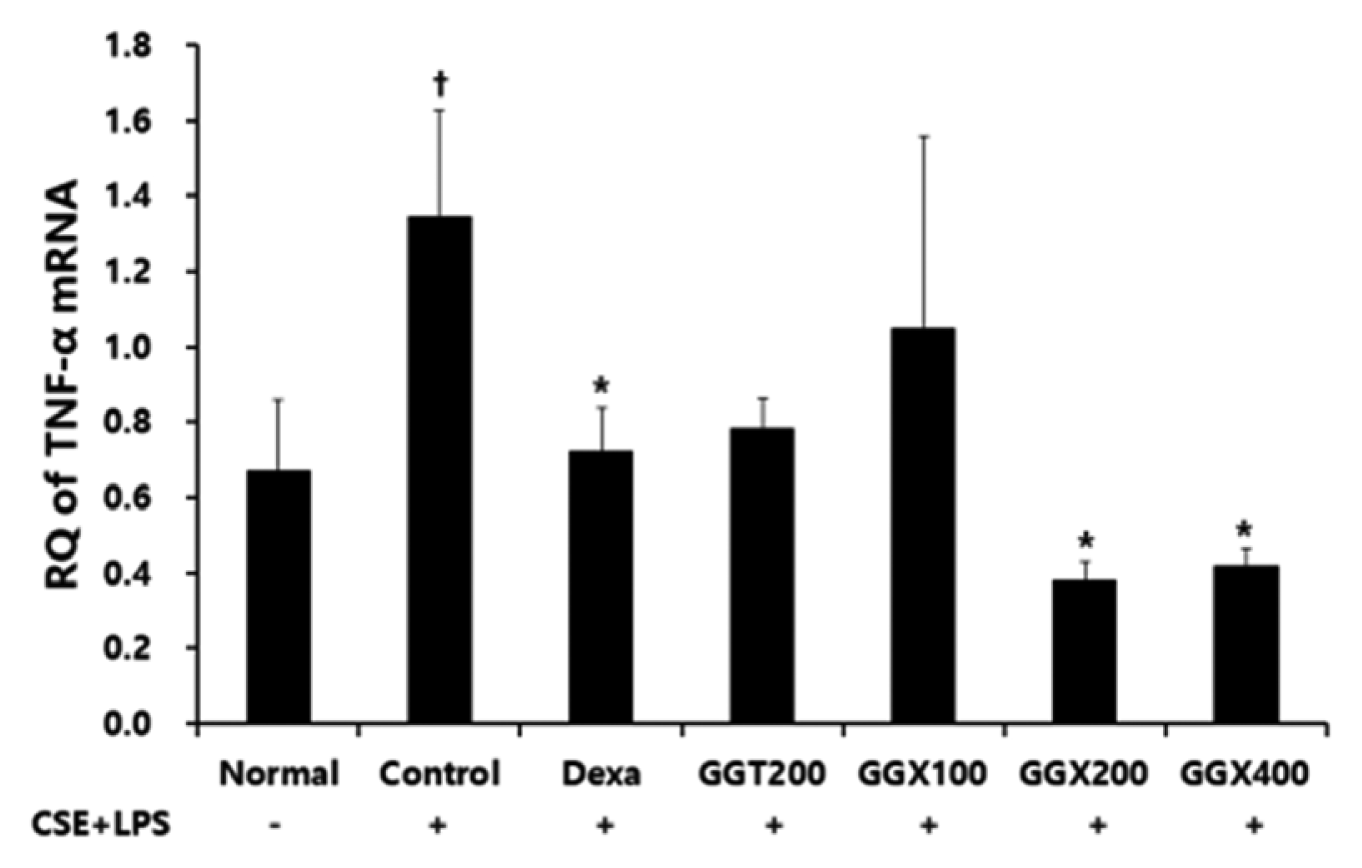
Effect of GGX on TNF-α mRNA expression in lung tissue of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=4). Level of TNF-α was determined with real time PCR. All values are presented as mean±SE. †: Significant difference with the non-treated group († p<0.05), *: Significant difference with the Control (* p<0.05).
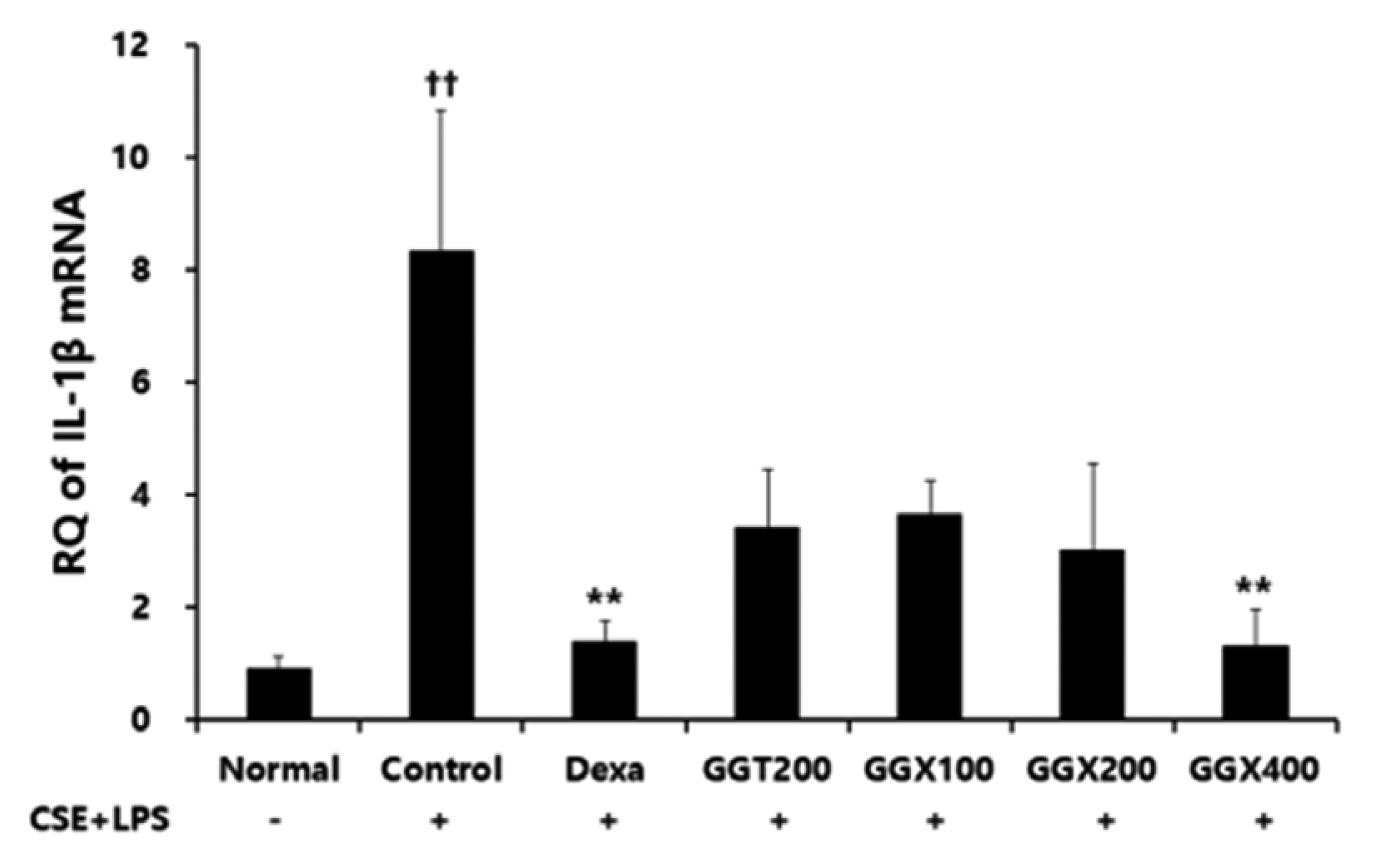
Effect of GGX on IL-1β mRNA expression in lung tissue of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=4). Level of IL-1β was determined with real time PCR. All values are presented as mean±SE. †: Significant difference with the non-treated group (†† p<0.01), *: Significant difference with the Control (** p<0.01).
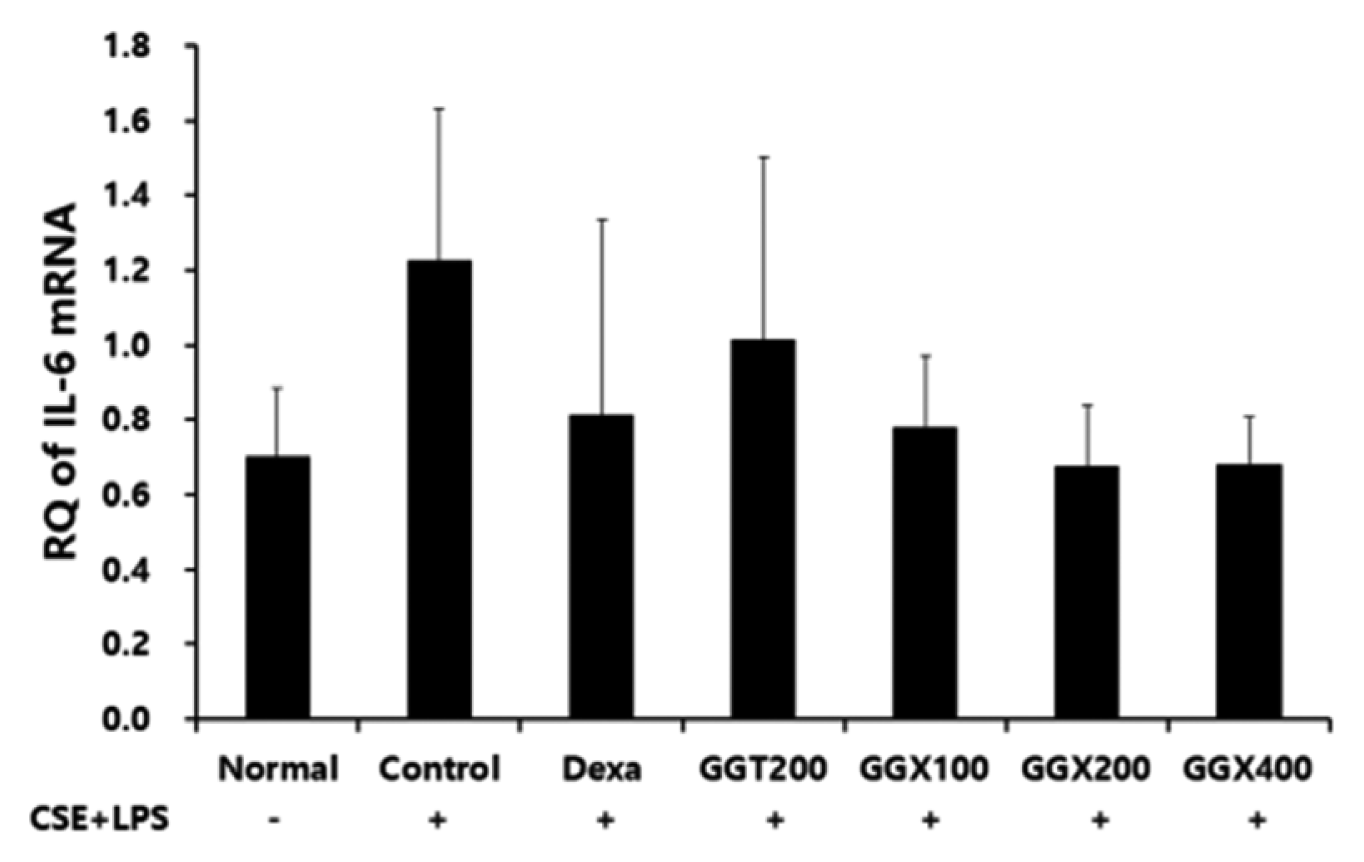
Effect of GGX on IL-6 mRNA expression in lung tissue of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=4). Level of IL-6 was determined with real time PCR. All values are presented as mean±SE.
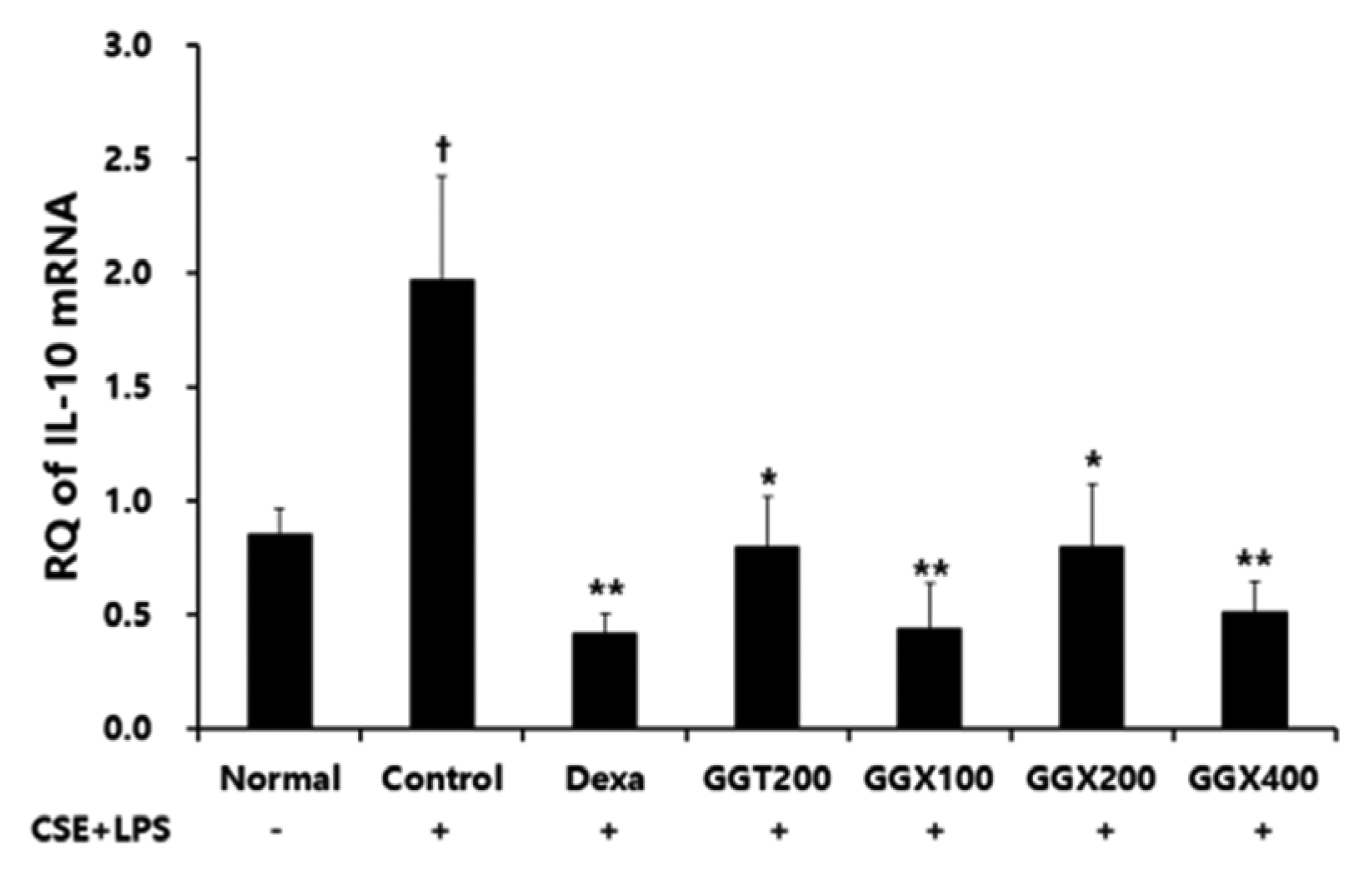
Effect of GGX on IL-10 mRNA expression in lung tissue of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=4). Level of IL-10 was determined with real time PCR. All values are presented as mean±SE. †: Significant difference with the non-treated group († p<0.05), *: Significant difference with the Control (* p<0.05, ** p<0.01).
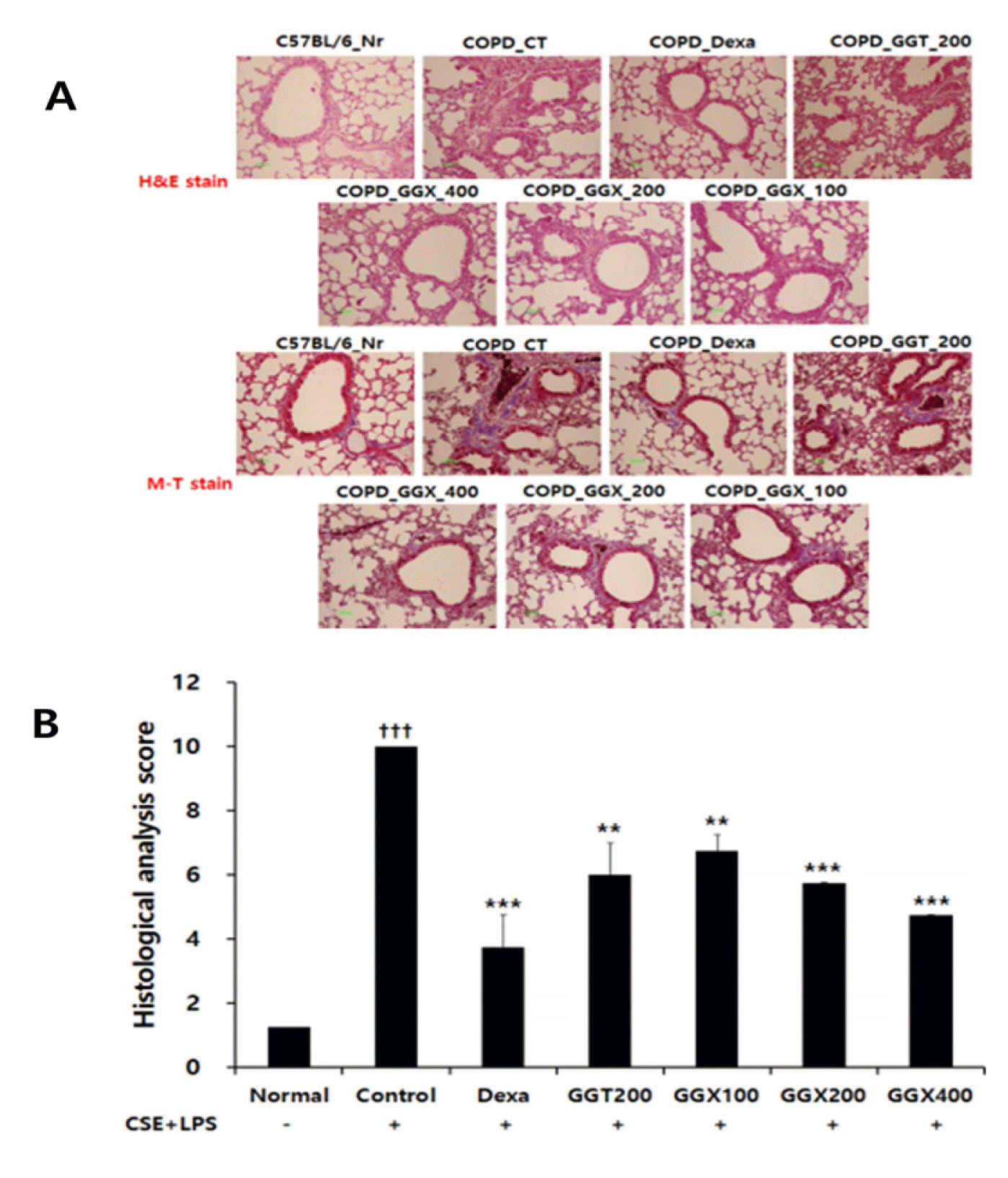
Effect of GGX on histopathological changes and histology scores in the lung of COPD mice.
Mice were challenged by an aspiration of LPS+CSE (Control), and treated with Dexa (dexamethasone 3 mg /kg), GGT (200 mg /kg) and GGX (100, 200, 400 mg /kg) for 21 days (n=4). (A) Representative sections from each treatment group are shown (Light microscope at 100×magnification). (B) Quantitative analysis of the degree of lung tissue damage in the sections. All values are presented as mean±SE. †: Significant difference with the non-treated group (††† p<0.001), *: Significant difference with the Control (** p<0.01, *** p<0.001).