Assessment of genotoxicity of Ssanghwa-tang, an herbal formula, by using bacterial reverse mutation, chromosome aberration, and in vivo micronucleus tests
Article information
Abstract
Objectives
Ssanghwa-tang (SHT) is a traditional herbal formula comprising nine medicinal herbs, and it is used for reducing fatigue in Korea. SHT exerts various effects such as anti-inflammatory, antioxidant, and anti-aging activities, and protection against acute hepatotoxicity. However, the genotoxicity of SHT has not yet been established.
Methods
Ten components were identified in SHT water extract by using high-performance liquid chromatography analysis. We assessed the genotoxicity of SHT by using bacterial reverse mutation (Ames test), chromosome aberration, and in vivo micronucleus tests.
Results
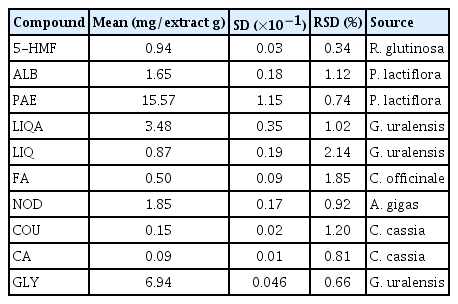
The contents of paeoniflorin, glycyrrhizin, and liquiritin apioside in SHT were 15.57, 6.94, and 3.48 mg/g extract, respectively. SHT did not increase the revertant colonies of Salmonella typhimurium and Escherichia coli strains in the presence or absence of metabolic activity. Although SHT did not induce structurally abnormal chromosomes in Chinese hamster lung (CHL) cells in the presence of metabolic activity, the number of structurally aberrated chromosomes increased dose-dependently in the absence of metabolic activity. In the in vivo micronucleus test, SHT did not affect the formation of micronuclei compared with the vehicle control.
Conclusions
Genotoxicity of SHT was not observed in the Ames test and in vivo micronucleus test. However, based on the results of chromosome aberration test, it can be presumed that SHT has the potential to induce genotoxicity because it induced structurally abnormal chromosomes in the absence of metabolic activity.
Introduction
Herbal medicines have been traditionally used for a long time and are now widely used to replace pharmaceutical drugs. The use of herbal medicines for the prevention and treatment of several diseases is expanding rapidly worldwide1). Herbal medicines have long been considered safe. However, no experimental basis has been established to determine the toxicity of traditional herbal medicines2). Recently, there have been increasing concerns on various toxicities, such as hepatotoxicity and nephrotoxicity, caused by traditional herbal medicines. Therefore, it is necessary to perform experiments to establish the basic safety of various herbal medicines3–7).
Ssanghwa-tang (SHT), a traditional herbal formula, has long been used in Korea for the reduction of fatigue caused by colds and for the enhancement of physical strength. Korean pharmaceutical companies produced 39 million bottles of SHT in 2016, and it was in the top 100 over-the-counter drugs8). In recent studies, SHT has been reported to possess many functions, such as protection against acute hepatotoxicity, anti-melanogenic activity, and inhibitory effects on bone loss9–11). It has also been reported to exert analgesic, anti-convulsant, anti-oxidative, anti-aging and anti-inflammatory effects12–14). A recent study showed that SHT exerted anti-inflammatory effects on cigarette smoke-induced airway inflammation by modulation pro-inflammatory cytokines through the matrix metallopeptidase 9 (MMP-9) and extracellular signal-regulated kinase (ERK) signaling pathways15). Although various effects of SHT have been identified, studies on its toxicity have not been performed sufficiently. The acute and repeated oral dose toxicity studies of SHT have been archived in previous reports16,17), but the genotoxicity has not yet been studied.
Genotoxicity test is performed to confirm that there is no possibility of carcinogens or mutagens. Therefore, in this study, we aimed to test the genotoxicity of SHT by using bacterial reverse mutation (Ames test), chromosome aberration, and in vivo micronucleus tests. These tests were performed according to the Organization for Economic Cooperation and Development (OECD) guidelines18–20).
Materials & Methods
1. Chemicals and reagents
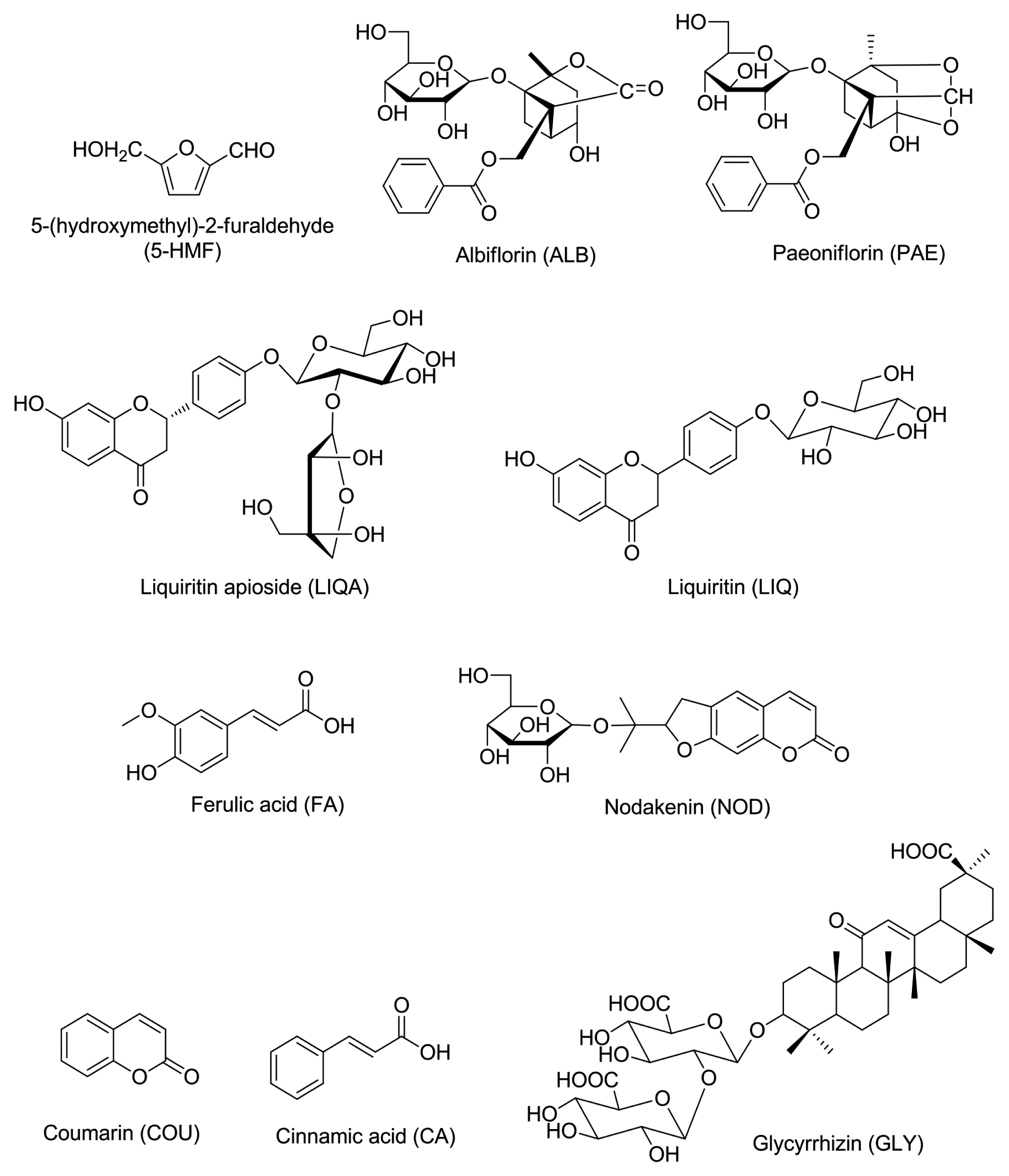
Albiflorin (ALB, 99.8%), paeoniflorin (PAE, 98.8%), ferulic acid (FA, 98.0%), liquiritin (LIQ, 99.6%), and glycyrrhizin (GLY, 99.0%) were purchased from Wako Pure Chemical (Osaka, Japan). Cinnamic acid (CA, 99.0%), coumarin (COU, 99.0%), and 5-(hydroxy-methyl)furfural (5-HMF, 99.0%) were acquired from Merck KGaA (Darmstadt, Germany). Liquiritin apioside (LIQA, 98.0%) and nodakenin (NOD, 99.5%) were obtained from Shanghai Sunny Biotech (Shanghai, China) and ChemFaces Biochemical (Wuhan, China), respectively. High-performance liquid chromatography (HPLC)-grade solvents (methanol, acetonitrile and water) were purchased from J.T. Baker (Phillipsburg, NJ). Anhydrous acetic acid (glacial) for HPLC was ACS reagent grade and purchased from Merck KGaA (Darmstadt, Germany).
2. Plant materials
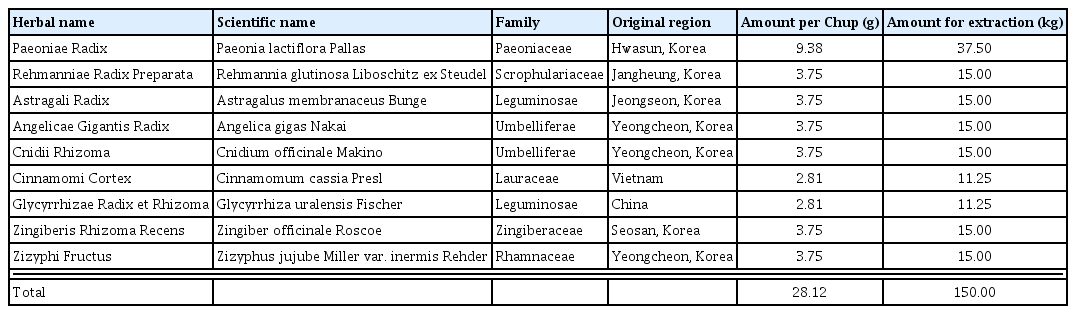
The nine raw materials (Table 1) of SHT decoction were obtained from Kwangmyungdang Medicinal Herbs (Ulsan, Korea) in October 2016, and identified by Dr. Goya Choi of the Korea Institute of Oriental Medicine (KIOM). The voucher specimens (2016–KE21–1–KE21–9) have been stored at KIOM.
3. Preparation of Ssanghwa-tang water extract
To obtain the SHT decoction extract, the nine raw materials described in Table 1 were mixed and extracted with distilled water (DW, 1,500 L) at 80°C for 2 h using the reflux method in Sungil Bioex Co., Ltd. (Hwaseong, Korea). The liquid extract was spray-dried to obtained powdered extract of 39.0 kg (extraction yield: 26.0%).
4. HPLC analysis of Ssanghwa-tang decoction
Analysis of the 10 marker components (Fig. 1), ALB, PAE, FA, LIQ, GLY, CA, COU, 5-HMF, NOD, and LIQA, in Ssanghwa-tang was conducted using the Shimadzu Prominence LC-20A series HPLC system (Kyoto, Japan) equipped with a photodiode array (PDA) detector and LCsolution (Version 1.24, SP1, Kyoto, Japan) for measurement and processing of chromatographic data. The 10 components were separated on a Phenomenex Gemini C18 column (250 × 4.6 mm, 5 μm particle size, Torrance, CA, USA) at 40°C. The mobile phases were 1.0% (v/v) acetic acid in DW (A) and 1.0% acetic acid in acetonitrile (B). The gradient flow conditions were as follows: 5–60% B for 0–40 min, 60–100% B for 40–45 min, 100% B for 45–50 min, and 100–5% B for 50–55 min. The analysis was performed at a flow rate of 1.0 mL/min and an injection volume of 10 μL.
5. Preparation of S9 mixture
Many materials may induce mutagenicity after metabolism, even if they are not mutagenic before metabolism in the body. To confirm metabolism-induced mutagenicity, in vitro genotoxicity tests were performed by adding metabolic activation system extracted from the liver of rat. The S9 mixture was prepared by mixing the exogenous metabolizing system (S9 fraction) and cofactor. The S9 fraction was obtained from Molecular Toxicology, Inc. (Boone, NC, USA). The final concentration of cofactors was 8 μM MgCl2, 33 μM KCl, 5 μM glucose-6-phosphate, 4 μM nicotinamide adenine dinucleotide phosphate (NADPH), 4 μM nicotinamide adenine dinucleotide (NADH), and 100 μM sodium phosphate buffer.
6. Bacterial reverse mutation test (Ames test)
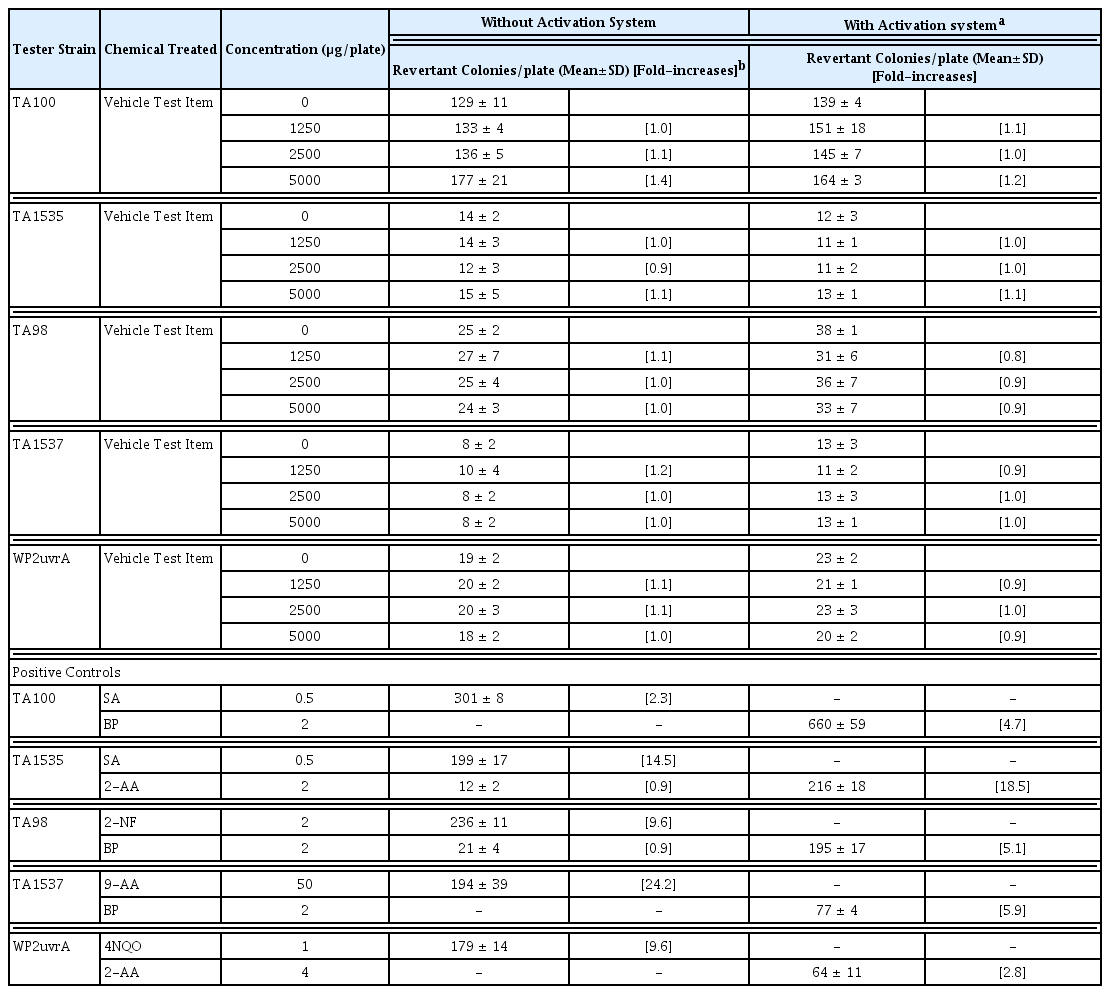
The Ames test was carried out according to OECD guideline18). The experiment was performed by the method of Maron and Ames21). Histidine-requiring Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537, and the tryptophan-requiring Escherichia coli strain WP2uvrA are known to be sensitive to mutagens and are commonly used in bacterial mutagenicity studies. All strains were obtained from Molecular Toxicology, Inc. (Boone, NC, USA). SHT was dissolved in DW and the maximum treated concentration was 5000 μg/plate, as determined by OECD guidelines. Each strain was treated at concentrations of 0, 1250, 2500, and 5000 μg/plate in the presence or absence of S9 mix. Sodium azide (SA), 2-nitrofluorene (2-NF), 9-aminoacridine (9-AA), 4-nitroquinoline N-oxide (4NQO), 2-aminoanthracene (2-AA), and benzo (a)pyrene (BP) were respectively used as positive controls. The results are presented as the mean of the number of revertant colonies that occurred at each concentration three times on each plate with the standard deviation (mean ± SD).
7. Chromosome aberration test
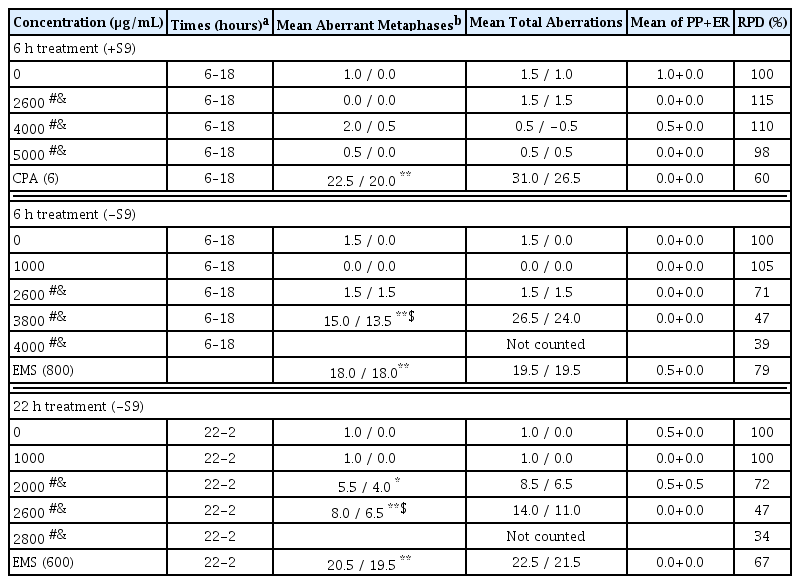
The chromosome aberration test was conducted in accordance with OECD guideline19), using the method described previously, with minor modifications22–24). Chinese hamster lung (CHL) cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The number of chromosomes in CHL cells was 25 and the doubling time was approximately 15 h. The cells were cultured at 37°C in a 5% CO2 atmosphere in minimum essential medium containing 10% fetal bovine serum, 2.2 g/L of sodium bicarbonate, 2 mM of L-glutamine, 100 μg/mL of streptomycin sulfate and 100 units/mL of penicillin G·Na. The concentration of SHT was determined based on solubility and cytotoxicity in a preliminary range-finding study. SHT 5000 μg/ml was used as the highest concentration and serially diluted twofold to a final concentration of 19.53 μg/mL. The cells were treated with SHT in the presence (+S) and absence (−S) of S-9 mix. The relative population doubling (RPD) was calculated and used as an index of cell proliferation. In the presence of S9 mixture, the concentrations of SHT were 2600, 4000, and 5000 μg/mL for 6 h. In the absence of S9 mixture, the concentration of SHT was 1000, 2600, 3800, and 4000 μg/mL and 1000, 2000, 2600, and 2800 μg/mL for 6 and 22 h, respectively. After completion of treatment with SHT, the cells were washed with Ca++ and Mg++ free Dulbecco’s phosphate buffered saline (CMF D-PBS) and fresh medium was added. SHT, positive control, and vehicle control (DW) were treated for 6 h with or without S9 mixture, and 22 h without S9 mixture. Ethyl methanesulfonate (EMS) was used as a positive control in the absence of S9 mixture, and cyclophosphamide monohydrate (CPA) was used as a positive control in the presence of S9 mixture. After incubation, the cells were fixed in fixation solution (methanol:glacial acetic acid = 3:1, v/v) for 20 min at 4°C and replaced with fresh fixation solution after centrifugation at 1000 rpm for 5 min. The fixed cells were dropped on slides and stained with 3% (v/v) Giemsa solution prepared with Gurr buffer. The chromosomal aberrations were differdentiated and counted according to the ‘Atlas of chromosome aberration by chemicals (JEMS-MMS, 1988)’25).
8. In vivo micronucleus test
The in vivo micronucleus test was performed according to OECD guideline20). Six-week-old male ICR specific-pathogen-free mice were obtained from Orient Co., Ltd. (Seongnam, Korea). The mice were housed 3 per cage and maintained at a temperature of 22±3°C, relative humidity of 40–70%, 12 h light/dark cycle, light intensity of 150–300 Lux, and air ventilation frequency of 10–20 times/h. The highest SHT concentration was set at 2000 mg/kg, and SHT was orally administered for 2 days at concentrations of 500, 1000, and 2000 mg/kg, respectively. CPA (Sigma-Aldrich, USA) was used as a positive control. It was intraperitoneally administered at 70 mg/kg. After 23 h from the final administration, the mice were sacrificed by CO2 asphyxiation, and bone marrow cells were separated and stained with 5% (v/v) Giemsa solution26). Circular and oval bodies showing the same staining pattern as the nuclei of surrounding nucleated cells and with size ranging from 1/5–1/20th of the diameter of the cell were counted as micronucleus (MN). The number of micronucleated polychromatic erythrocytes (MNPCEs) among 2000 polychromatic erythrocytes (PCEs) per individual was measured and expressed as the induced frequency of micronucleus. The ratio of PCEs/[PCEs + normochromatic erythrocytes (NCEs)] was measured by counting 500 erythrocytes regardless of micronucleus.
The in vivo micronucleus test protocol was approved by the Institutional Animal Care and Use Committee of Korea institute of Toxicology (approval number: 1706-0244).
Results
1. Marker components of SHT
In the present study, an optimized HPLC–PDA method was successfully applied for the quantitative analysis of the 10 main components in SHT. All the components were separated within 45 min. Representative, HPLC chromatograms of the standard mixtures and SHT sample are shown in Fig. 2. For the quantitative analysis of each compound, ultraviolet absorbance was monitored at the following wavelengths: 230 nm for ALB and PAE; 254 nm for GLY; 275 nm for LIQA, LIQ, COU, and CA; 280 nm for 5-HMF; 320 nm for FA; and 335 nm for NOD, respectively. The quantity of the 10 marker components in SHT sample was in the range of 0.09–15.57 mg/g of the extract (Table 2).
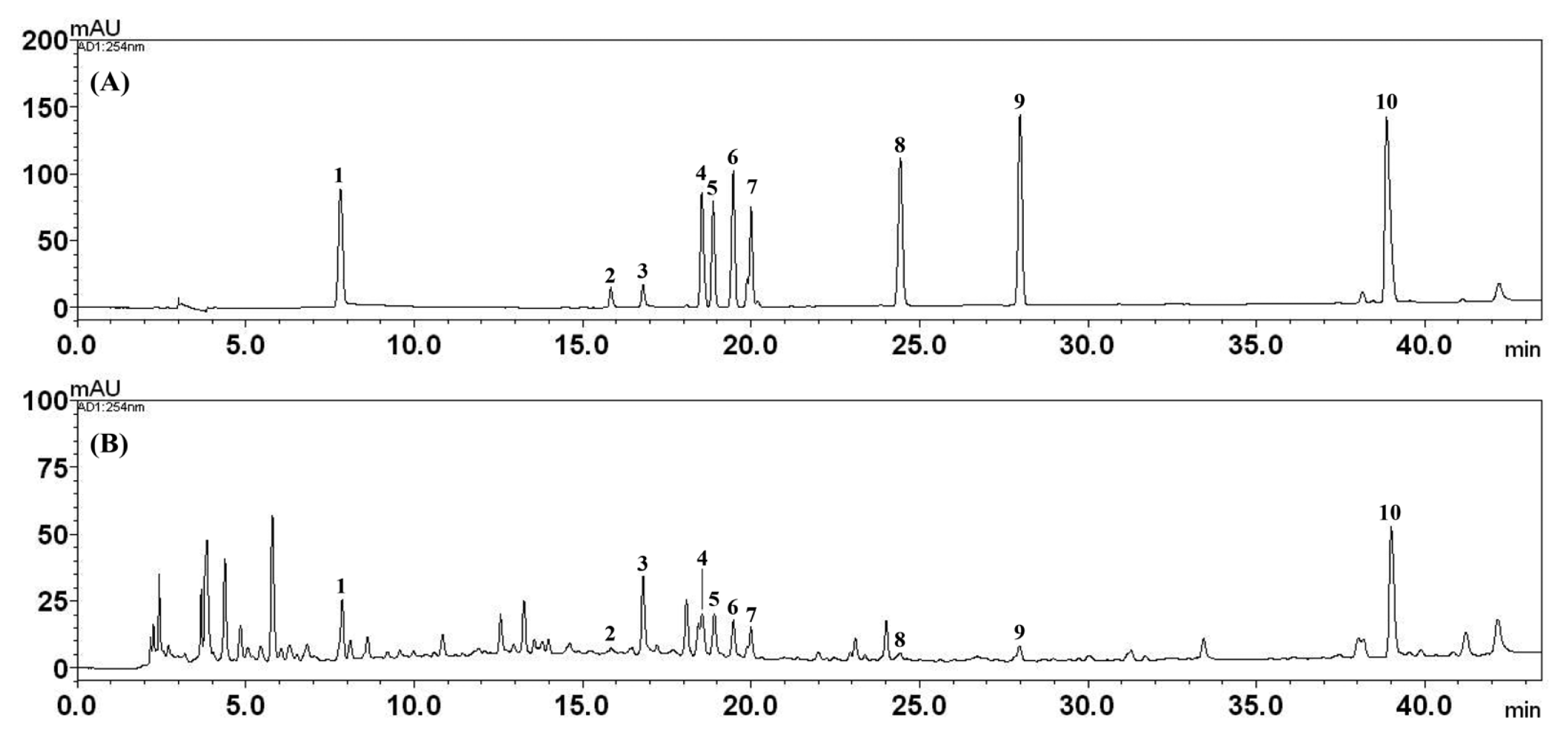
Representative HPLC chromatogram of the standard mixture (A) and Ssanghwa-tang decoction (B) at 254 nm. 5-HMF (1), ALB (2), PAE (3), LIQA (4), LIQ (5), FA (6), NOD (7), CA (8), COU (9), and GLY (10).
2. Ames test
In the S. typhimurium strains TA98, TA100, TA1535, and TA1537 and E. coli strain WP2uvrA, no reverse mutation was observed in any concentration (1250, 2500, and 5000 μg/mL) groups treated with SHT in the presence or absence of metabolic activation (Table 3). The positive controls significantly increased the number of revertant colonies, indicating that the test was valid. This result showed that SHT has no mutagenic or carcinogenic potential.
3. Chromosomal aberration test
In the preliminary dose range-finding study, no cytotoxicity was observed when CHL cells were treated with SHT for 6 h in the presence of metabolic activity. In the absence of metabolic activity, treatment with SHT at a concentration of 3800 μg/mL for 6 h and 2600 μg/mL for 22 h resulted in approximately 50% cytotoxicity (6-S, RPD 51%; 22-S, RPD 50%, Supplementary Table 1 and 2). Turbidity was observed at a concentration greater than 1250 μg/mL in all conditions. In the presence of metabolic activity (+S), SHT showed no significant increases in the numerical aberrant metaphase cells and frequency of metaphases with structural aberration compared with the vehicle control. In the absence of metabolic activity, there were significant increases in the frequency of metaphases with structural aberration depending on the concentration of SHT and duration (6 or 22 h) compared with the vehicle control (Table 4). There was no observable increase the number of aberrant metaphase cells. The positive controls significantly increased the frequency of metaphases with structural aberration, suggesting that the test was valid.
4. Micronucleus test
Body weight showed no significant changes in mice administered SHT compared with that in mice administered vehicle control (Supplementary Fig. 1). Moreover, there were no deaths and adverse clinical signs associated with SHT in mice but also no significant increase in the number of MNPCEs and ratio of PCEs/(PCEs + NCEs) at any dose of SHT (Table 5). This test was appropriated because the number of MNPCEs and ratio of PCEs/(PCEs+NCEs) increased in the CPA-treated group, which was a positive control.
Discussion
In traditional medicine, medicinal herbs have been used for centuries. In recent years, its use has been increasing as an alternative or supplement to counter the limitations of modern medicines. Consumers expect herbal medicines to be more user-friendly and safer than conventional drugs such as synthetic chemicals because these originate from nature and have many clinical applications. However, some herbal medicines contain toxic ingredients that cause serious adverse events. We investigated such medicines to establish safety data for widely used herbal formulas in Korea. In previous studies, we have reported the acute and sub-acute toxicity of SHT in rats. There was no death or any adverse clinical signs at the highest dose of SHT (2000 mg/kg) in both male and female rats. In the 4-week repeated oral administration toxicity study of SHT, no adverse signs were observed at the dose 2000 mg/kg, while minor changes were observed at the highest dose of 5000 mg/kg. In the present study, the genotoxicity of SHT was estimated by bacterial reverse mutation, in vitro chromosomal aberration and in vivo micronucleus formation tests. The maximum dose of SHT for the micronucleus formation test in mice was 2000 mg/kg, which converts to a human equivalent dose of 9,756 mg/60 kg adult27). In humans, the dose of 1 Chup of SHT is 28.12 g as a herbal mixture before extraction (Table 1), and when the extraction yield (26.0%) is reflected, it corresponds to 7,311 mg as an extract powder.
Genotoxicity includes mutagenicity and carcinogenicity according to DNA damage. The Ames test is a bacterial reverse mutation assay that can be performed in a short period to confirm whether a substance has the potential to cause gene mutations through genetic damage28). The in vitro mammalian chromosome aberration test was conducted to detect the structural chromosomal aberrations29). The in vivo micronucleus test is a method to confirm genotoxicity by detecting chemical substance-induced micronucleus. Micronuclei are generated by chromosome aberrations, defects of the cell repair system, and accumulation of DNA damage. The formation of micronucleus induced by substances with genotoxicity can lead to cell death or cancer30).
In the present study, SHT did not cause reverse mutagenesis in all five bacterial strains, nor did it induce micronucleus formation in mice. In contrast, in CHL cells, chromosomal abnormalities were induced by more than 1000 μg/mL of SHT. In a previous study, Paeoniae Radix, Rehmanniae Radix Preparata, Angelica Gigantis Radix, Cnidii Rhizoma, and Cinnamomi Cortex showed negative results for the chromosomal aberration test in the absence of metabolic activation system in CHL cells at 100 μg/mL extract concentration. On the other hand, Paeoniae Radix, Angelica Gigantis Radix, and Cnidii Rhizoma in the presence of the metabolic activation system induced chromosomal abnormalities in 9%, 7%, and 6% of the observed cells, respectively31). The conditions that induced chromosomal abnormalities in CHL cells by SHT were the absence of metabolic activation system and more than 1000 μg/mL of SHT. It means that SHT itself, not metabolites of SHT, causes chromosomal abnormalities. Also, when SHT taken at normal dose, a concentration of 1000 μg/mL is considered too high to reach in the body. Chromosomal aberration can be caused by a mechanism related to cytotoxicity even by drugs that do not attack DNA, and it is known that the in vitro chromosomal aberration test has a high possibility of showing ‘false positive’ by inducing cytotoxicity of the treated drug32). In addition, there is also a study that confirmed the plasma levels of chemicals which showed negative results in the Ames test and in vivo micronucleus test, but showed positive results in the in vitro chromosomal aberration/micronucleus test. According to the study, when the maximum concentration in plasma (Cmax) of the chemical in the in vivo test was lower than the lowest positive concentration (LPC) of the chemical in the in vitro test, negative results were obtained in the in vivo test33). There is a limitation to this study. It is difficult to confirm that this result alone is indicative of genotoxicity.
Conclusions
SHT did not cause genotoxicity in the Ames test and in vivo micronucleus test, but it caused chromosomal abnormalities in CHL cells. We suggest that although SHT showed a positive result in the in vitro chromosomal aberration test, 1) because the concentration of SHT inducing positive chromosomal aberration showed about 50% cytotoxicity in CHL cells, it may be a ‘false positive’ and 2) because SHT did not induce micronucleation in mice, the Cmax of SHT in mice may be lower than the LPC of SHT in CHL cells. Additional tests such as comet assay should be performed to ensure genotoxicity.
Supplementary Information
Acknowledgements
This research was supported by grants of the ‘Construction of Scientific Evidence for Herbal Medicine Formulas (K17251)’ and ‘Construction of Safety and Efficacy for Traditional Herbal Prescriptions of Medicinal Institution (KSN2013310)’ projects from the Korea Institute of Oriental Medicine (KIOM), Republic of Korea. We would like to thank the Korea Institute of Toxicology (KIT) for providing animal care facility for the study.
Notes
Conflict of interest
The authors declare that they have no conflict of interest.