A traditional East Asian herbal complex, Majainhwan for constipation in Parkinson’s disease: A retrospective study
Article information
Abstract
Objectives
Parkinson’s disease (PD) patients experience various non-motor symptoms, among which constipation is the second most common after anosmia. However, there are no clear guidelines or effective treatment for constipation in PD.
Methods
To investigate the efficacy of Majainhwan (MH) on constipation in patients with PD, we conducted a retrospective chart review study of PD or Parkinsonism patients with constipation who received outpatient or inpatient treatment and whose previous laxatives were replaced by MH from August 2016 to July 2019.
Results
In this study, a total of 68 patients’ medical records were reviewed. Among the 44 outpatients with MH treatment, “effective” results were observed in 86.4% patients. Similarly, among the 24 inpatients, “effective” results were noted in 95.8% patients. The adverse effect was “diarrhea” reported in five cases.
Conclusion
Based on these findings, we could suggest that MH is relatively safe and may be effective in the treatment of constipation in patients with PD.
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disease evidenced by motor symptoms, such as resting tremor, rigidity, bradykinesia, and postural instability, and characterized by the loss of dopaminergic neurons in the substantia nigra1).
While PD is characterized by motor symptoms, there are various clinically important non-motor symptoms (NMS), including autonomic ataxia (gastrointestinal system, genitourinary system, pupil movement, cardiovascular system, body temperature regulation, and sexual function), sleep disturbance, neuropsychiatric complications, pain, and fatigue2,3]. Recently, several lines of evidence have shown that NMS can adversely affect the quality of life (QoL) in patients with PD4). In a recent meta-analysis, the prevalence of constipation in patients diagnosed with PD was 50%, confirming it as the second most common NMS after olfactory impairment, with approximately 20% of all patients having constipation symptoms before a PD diagnosis5). Another meta-analysis reported that people with constipation had a higher risk of developing PD than those without, and that constipation occurred 10 years before PD diagnosis6).
Meanwhile, changes in the colon, such as constipation, are considered a potential factor leading to degenerative brain disease through gut-brain interaction7) or by the dual-hit hypothesis8). Several studies have demonstrated differences in gut flora composition between patients with PD and people without PD, including studies on the interaction between gut flora and antiparkinsonian drug metabolism, such as changes in gut flora due to gastrointestinal side effects of catechol-o-methyl transferase (COMT) inhibitors and anticholinergics9); the relationship between gut flora and the levodopa drug metabolism10) have been reported, drawing attention to the gut flora in patients with PD.
Emphasizing the frequency of defecation, constipation is medically defined as <1 defecation every three to four days, whereas the Rome III criteria revised in 2006 includes the degree of subjective symptoms11). Chronic constipation can be diagnosed with reference to the Rome III for diagnosis of functional constipation, and the Bristol Stool Form Scale after identifying the organic causes by tests, such as digital rectal examination (DRE) and colonoscopy based on medical history12).
Constipation is one of the most common PD prodromes and NMS13), and causes serious functional impairment14). However, existing Western medical treatments and drugs alone seem to lack a beneficial effect on changes in the intestinal environment, such as improvement of constipation symptoms. Magnesium oxide (MgO), recommended as a first-line treatment for chronic constipation, is known to lead to hypermagnesemia15), whereas MgO co-administration with levodopa is known to decrease levodopa blood levels16).
Reports on the effectiveness of herbal medicine prescriptions for constipation in patients with PD or Parkinsonism have emerged since 2000, including related to Daikenchuto17) and Majainhwan (Mashiningan in Japanese, MaZiRenWan in Chinese)18) in Japan. Until now, only a few case reports19–20) employing Sasang constitutional medicine which is a unique system of traditional Korean medicine have been identified as relevant in South Korea. Therefore, this study aimed to test the therapeutic potential of Korean medicine, including herbal medicine, for patients with PD constipation. This study retrospectively analyzed the medical records of outpatients and inpatients at the Department of Cardiology and Neurology, College of Korean Medicine, Kyung Hee University Korean Medicine Hospital, and explored the efficacy and safety of Majainhwan on constipation in patients with PD.
Materials and Methods
1. Inclusion criteria
The medical records of outpatients or inpatients at the Department of Cardiology and Neurology of Kyung Hee University Korean Medicine Hospital from August 1, 2016, to July 31, 2019, meeting the following criteria were selected as study subjects.
Patients aged 19 years and older who complained of constipation (<1 defecation in three days) among those diagnosed with the Korean Standard Classification of disease and causes of death G20 Parkinson’s disease or G22 Parkinsonism.
Patients prescribed Majainhwan (in-hospital code: HH387) to control constipation symptoms.
Patients receiving additional treatment ≥1 after receiving Majainhwan prescription to identify changes in constipation symptoms.
2. Exclusion criteria
Patients meeting the following conditions were excluded from analysis:
Those who received additional treatment at least once, but had insufficient medical records to identify changes in constipation symptoms.
Patients who received treatment for PD at the first visit, but whose final diagnosis was a disease other than PD and Parkinsonism.
Patients who had other diseases during the study period and discontinued treatment.
3. Experimental medication
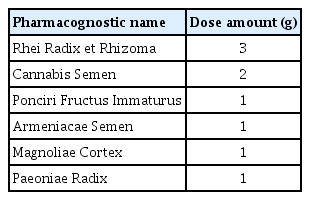
Majainhwan (in-hospital code: HH387) was prepared in the hospital according to the prescription book of Kyung Hee University Korean Medicine Hospital. Table 1 lists the composition and capacity of each pack.
The manufacturing process of Majainhwan is as follows:
Half the ingredient’s weight is weighed, extracted for 1.5 hour in distilled water 20 times the ingredient’s amount, concentrated, and dried to prepare an extract (A).
The remaining half of the ingredient is pulverized into a fine powder (B).
A and B are mixed, and uniformly molded into the size of a mung bean in an automatic pill-forming machine.
The pill is covered with a small amount of corn starch and placed in the pill rounder to form a smooth and even surface.
After drying for 10 hour, it is placed in the pill rounder for coating and dried to complete the process.
Dosing was one–three times per day, 0.5–1 pack each time, depending on the severity of symptoms and the presence of coexisting diseases. In advance, patients were asked whether they had been taking drugs other than Majainhwan to control constipation, and those taking other drugs for constipation were asked to stop those to restrict co-administration.
4. Data collection
Outpatient and inpatient records of the selected patients according to inclusion/exclusion criteria were examined to collect sociodemographic data, such as age and sex; PD-related data, such as duration of disease (years) and severity (Hoehn and Yahr stage); inpatient treatment status data; Majainhwan administration-related data, such as daily dose, frequency of administration per day, total time of administration (days), and adverse events; and data regarding the course of constipation symptoms, such as the number of bowel movements before treatment and that after treatment. The efficacy of Majainhwan was evaluated based on the collected data.
Observed items
1) Changes in defecation interval before and after Majainhwan administration
The defecation interval immediately before Majainhwan administration, as confirmed in the medical records, was defined as “defecation interval before treatment”, whereas that immediately after the final Majainhwan administration, as confirmed in the medical records, was defined as “defecation interval after treatment.” Defecation interval was marked as “number (n)” in “one defecation every n days.” For example, if “one defecation occurred in three days,” the defecation interval was marked as “3.”
2) Effectiveness of Majainhwan and the period for initial evaluation of effectiveness
Changes in defecation interval before and after treatment were examined, and if the interval decreased after treatment (a decrease in n) compared to before treatment, it was evaluated as “effective”; if there was no change in defecation interval, as “ineffective”; and if the defecation interval increased (an increase in n), as “worsened.”
If effective, the period (days) until the initial validity evaluation was confirmed in the medical record. According to the difference in medical record format between inpatients and outpatients, we evaluated inpatients and outpatients separately. For example, if a decrease in “n” was confirmed in the outpatient clinic seven days after the first dose in an outpatient who took Majainhwan for a total of 28 days, the period for initial judgment of effectiveness was “7 days.” However, since a daily defecation record was kept for inpatients, the period for initial evaluation of effectiveness (days) was calculated based on daily records.
3) Majainhwan administration and adverse effects
The dose of Majainhwan per administration (packs), number of doses per day (times), and the total period of administration (days) were investigated based on outpatient or inpatient medical records. When adverse effects were identified while taking Majainhwan, the details were recorded. The definition of diarrhea, one of the expected adverse effects, was based on the “Bristol Stool Form Scale,” which can estimate the duration of rectal pain by dividing it into Types 1 to 7 according to fecal consistency22).
4) Investigation of information related to PD and Parkinsonism
The time of onset and diagnosis of Parkinsonian symptoms were investigated based on outpatient or inpatient medical records, and the disease period (years) calculated. The Hoehn and Yahr stage was based on the patient evaluation at the first visit.
5) Investigation of coexisting diseases
Coexisting diseases identified in outpatient or inpatient medical records were investigated.
5. Statistical analysis
SPSS for Windows (version 18.0) was used for statistical analysis. The general characteristics and Hoehn and Yahr stages of the study subjects, corresponding to non-continuous variables, were described as “number (n)” and “percent (%)”. The occurrence of adverse effects and the presence of coexisting diseases are summarized as n (%) with the occurrence and presence status. Continuous variables were tested for normality using the Shapiro-Wilk test and described as mean±standard deviation (SD). To analyze the change in defecation interval before and after treatment, a paired t-test was used for normal distribution, and the Wilcoxon signed-rank test for non-normal distribution. To analyze the correlation between severity based on the Hoehn and Yahr stage and the effect of Majainhwan, the Fisher’s exact test was used when an expected frequency < 5 accounted for > 20% of the total data. Statistical significance was verified based on a p-value <0.05 in all analyses.
6. Ethical approval and protocol registration
The present study was designed in compliance with the Code of Ethics of the Helsinki Declaration (South Africa Amendment, 1996.10), the Korean Good Clinical Practice (KGCP), and related regulations, and approved by the Institutional Review Board (IRB) of Kyung Hee University Korean Medicine Hospital (KOMCIRB 2020-02-002). The protocol of this retrospective chart review was registered on Clinical Research Information Service (CRIS) 2020 (registration number: KCT0005033).
Results
From August 01, 2016, to July 31, 2019, there were 71 outpatients and 43 inpatients at the Department of Cardiology and Neurology, Kyung Hee University Korean Medicine Hospital, who were aged 19 years or older and received Majainhwan with prescription for treatment of constipation associated with PD (G20) and Parkinsonism (G22). Of these, 23 outpatients and 5 inpatients who had insufficient medical records or were difficult to follow up were excluded, as were 3 outpatients and 11 inpatients finally diagnosed with a disease other than PD and Parkinsonism or who had other diseases during the study period and discontinued treatment. In addition, one outpatient whose prescription was changed and three inpatients who did not meet the diagnostic criteria for constipation were excluded. Finally, the retrospective analysis was conducted based on the medical records of 44 outpatients and 24 inpatients to evaluate the efficacy of Majainhwan (Figure 1).
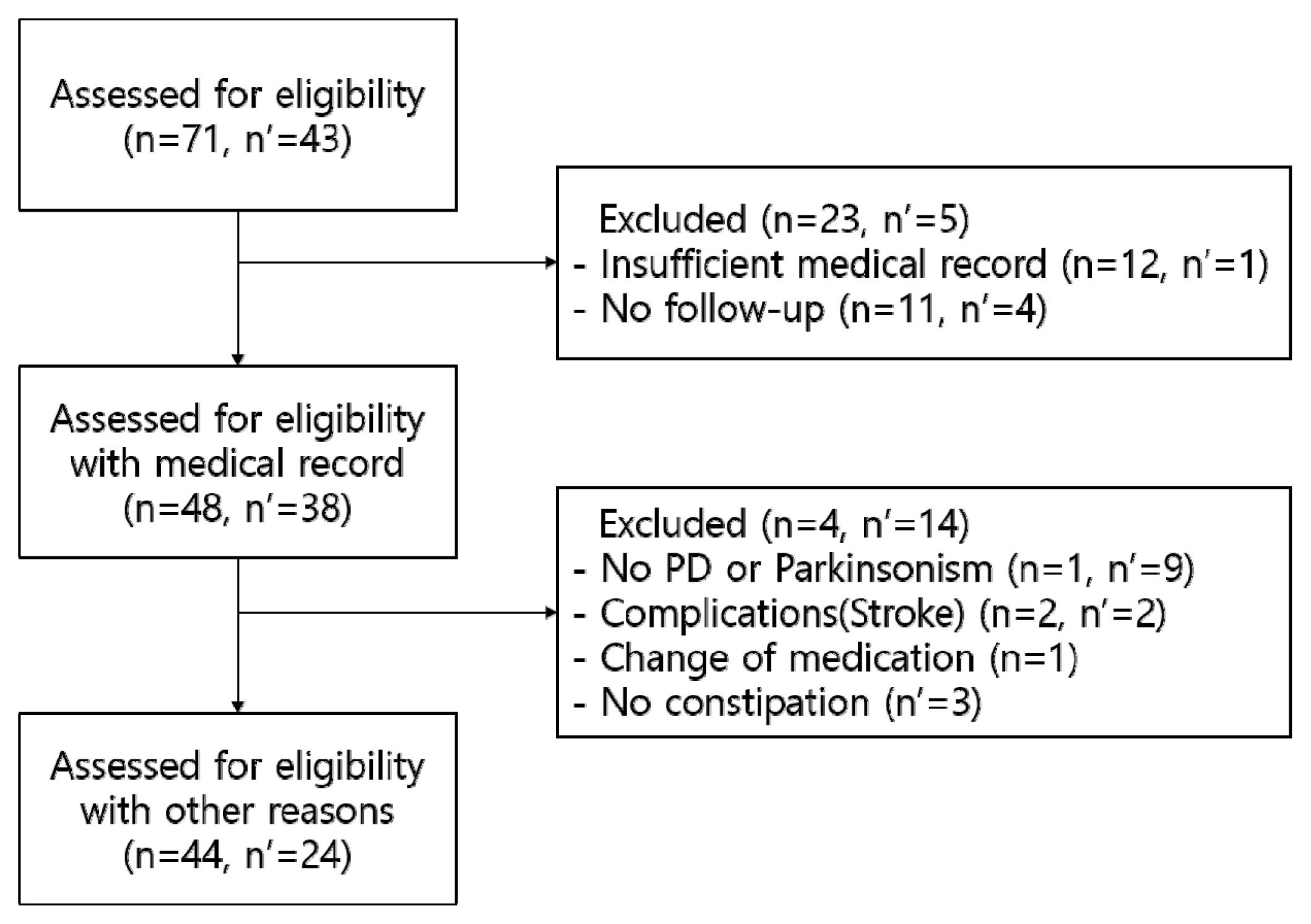
Flow chart of outpatient (n) & inpatient (n′) selection
PD: Parkinson’s disease, Cb.inf: Cerebral Infarction, Cb.hrr: Cerebral hemorrhage
1. Demographic and clinical characteristics
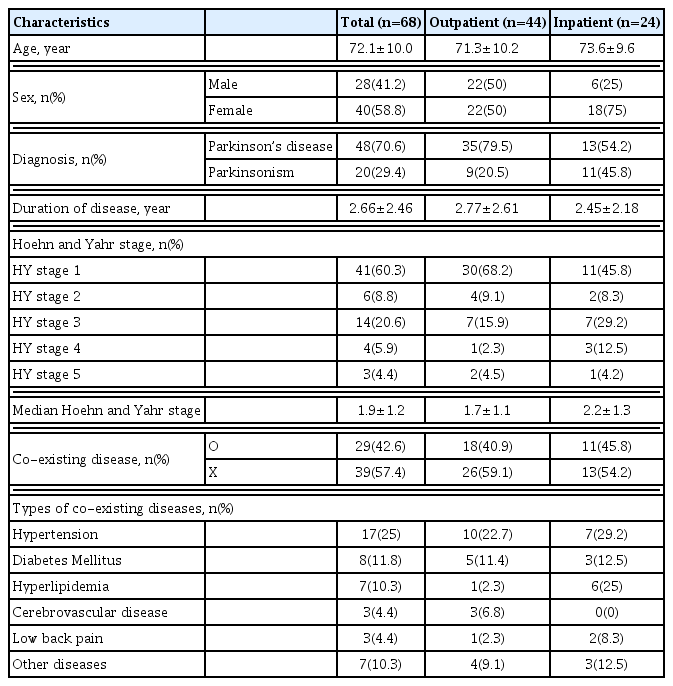
The demographic characteristics of 44 outpatients and 24 inpatients selected based on the inclusion and exclusion criteria of this study were analyzed retrospectively. The mean age of all patients was 72.1±10.0 years, and there were more female (40 patients, 58.8%) than male patients. The mean age of outpatients and inpatients was 71.3±10.2 and 73.6±9.6 years, respectively (Table 2).
There were 35 outpatients (79.5%) diagnosed with PD and 9 outpatients diagnosed with Parkinsonism (20.5%), whereas there were 13 in patients diagnosed with PD (54.2%) and 11 inpatients diagnosed with Parkinsonism (45.8%). The disease duration for outpatients and inpatients were 2.77±2.61 and 2.45±2.18 years, respectively. As for the Hoehn and Yahr stage, stage 1 accounted for most in both outpatients (30 patients, 68.2%) and inpatients (11 patients, 45.8%), followed by stage 3 with seven outpatients (15.9%), and seven inpatients (29.2%) (Table 2).
Among the study subjects, 18 outpatients (40.9%) and 11 inpatients (45.8%) had coexisting diseases. Prevalent coexisting diseases included hypertension (25%), diabetes (11.8%), hyperlipidemia (10.3%), and cerebrovascular diseases (4.4%) (Table 2).
2. Effectiveness evaluation
1) Changes in defecation interval before and after Majainhwan administration
The change in defecation interval before and after Majainhwan administration in outpatients was analyzed using the Wilcoxon signed-rank, whereas that in inpatients was analyzed by a paired t-test.
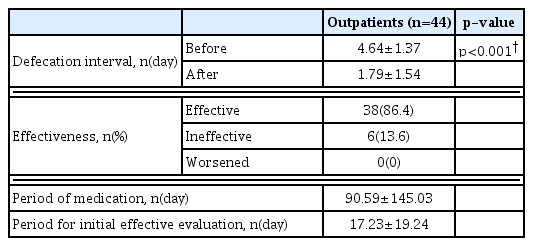
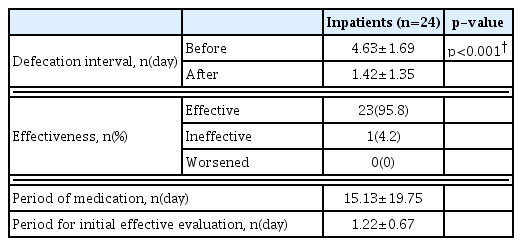
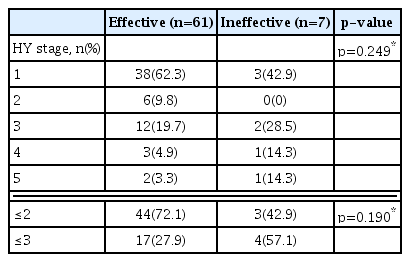
The defecation interval decreased after Majainhwan administration from “once every 4.64±1.37 days” to “once every 1.79±1.54 days” in outpatients, and from “once every 4.63±1.69 days” to “once every 1.42±1.35 days” in inpatients. A statistically significant decrease (p<0.05) in defecation interval was observed in both outpatients and inpatients (Table 3, and 4). No significant correlation between severity based on the Hoehn and Yahr stage and Majainhwan effect was identified (p>0.05) (Table 5).
2) Effectiveness of Majainhwan and the period for initial evaluation of effectiveness
Majainhwan was evaluated to be “effective” in 38 outpatients (86.4%) and 23 inpatients (95.8%); “ineffective” in six outpatients (13.6%) and one inpatient (4.2%); whereas none of the patients (0%) experienced “worsened” symptoms. The period for initial evaluation of effectiveness was 17.23±19.24 days for outpatients and 1.22±0.67 days for inpatients (Tables 3 and 4).
Discussion
This study conducted a retrospective analysis of 68 patients with PD or Parkinsonism who received Majainhwan for the treatment of constipation to investigate the demographic, clinical characteristics, safety, and adverse effects of Majainhwan. As a result of analyzing the difference in defecation interval before and after Majainhwan administration, Majainhwan was “effective” in 61 out of 68 patients (89.7%) with a statistically significant treatment effect (p<0.05), and relatively safe with only five cases of adverse effects (diarrhea, 7.35%), which may have resulted from the combination of various factors, such as dose, frequency of administration, and sensitivity to drugs. Despite these side effects, improved bowel movements will be advantageous in terms of QoL for patients with PD.
In this study, we employed Majainhwan as a cure for constipation in patients with PD based on the following evidence. Majainhwan is a prescription that first appeared in the “Synopsis of Prescriptions of the Golden Chamber”23). As a result of the lack of fluid and humor after cold damage, it was recommended for use in a disease pattern called splenic constipation, in which the stool hardens, making it difficult to defecate18). Patients with PD are mostly older adults24) and show progressive loss of weight with greater loss of both visceral and subcutaneous fat25), and are chronically in a state of physical decline and wasting. Considering these characteristics of patients with PD, we applied Majainhwan, which has been used for constipation in a physically exhausted state, to treat constipation in patients with PD.
Recently, a randomized clinical trial26) confirming the efficacy and safety of Majainhwan in the treatment of functional constipation was reported in China, and a study in Japan reported that Majainhwan was effective in improving opioid-induced constipation in vivo27). A previous case report in Japan also confirmed the efficacy and safety of Majainhwan in 23 patients with PD and constipation18). Compared to this Japanese report [18], the present study was conducted by dividing the 68 patients into inpatients and outpatients. In particular, the inpatients’ daily progress of treatment could be monitored, and the period until treatment effects clearly revealed.
However, this study has several limitations in that patients with insufficient medical records or who were not followed up were eliminated from analysis due to the retrospective nature of the chart review, and thus, the parameters for determining treatment effect and safety were limited. Accordingly, we could also not evaluate the pattern of stool or the degree of discomfort during defecation. During the chart review process, we found that the most existing medical records did not include evaluation tools such as the Bristol stool scale that can classify the form of stools. Second, we did not use laboratory test findings such as electrolyte tests in the safety evaluation. Therefore, the results of the present study on safety might be limited. Fourth, this study did not evaluate the influence of Majainhwan on dopamine blood levels. Fifth, the long-term improvement of symptoms following Majainhwan administration has not been evaluated. Finally, subjects of the study may have received treatment in conjunction with other Korean medicine treatments, including acupuncture. Therefore, it is necessary to conduct a comparative study by following up the study subjects to more accurately determine Majainhwan’s effects and safety in the future.
Nevertheless, this study is significant as it confirms the possibility of treatment with Majainhwan for constipation in patients with PD and Parkinsonism. Finally, this study could be a preliminary study laying the groundwork for various future studies, including a study on the effects and mechanisms of Majainhwan on the intestinal environment of patients with PD, a comparative study of the effects of Majainhwan and Western medicine on constipation in patients with PD, and a large-scale study to investigate the differences in the severity of constipation symptoms based on the Hoehn and Yahr stage and response to Majainhwan.
In conclusion, MH was found to be relatively safe and effective in the treatment of constipation in patients with PD.
Acknowledgments
This paper is based on Jun Ho Cho’s theses for Master’s Degree
Notes
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0147).