Severe Diarrhea-induced Acute Kidney Injury and Its Consequence in an Elderly
Article information
Abstract
Methods
This study presents a comprehensive case study of an elderly male diagnosed with acute kidney injury (AKI) resulting from severe dehydration, supported by an extended follow-up with laboratory findings.
Results
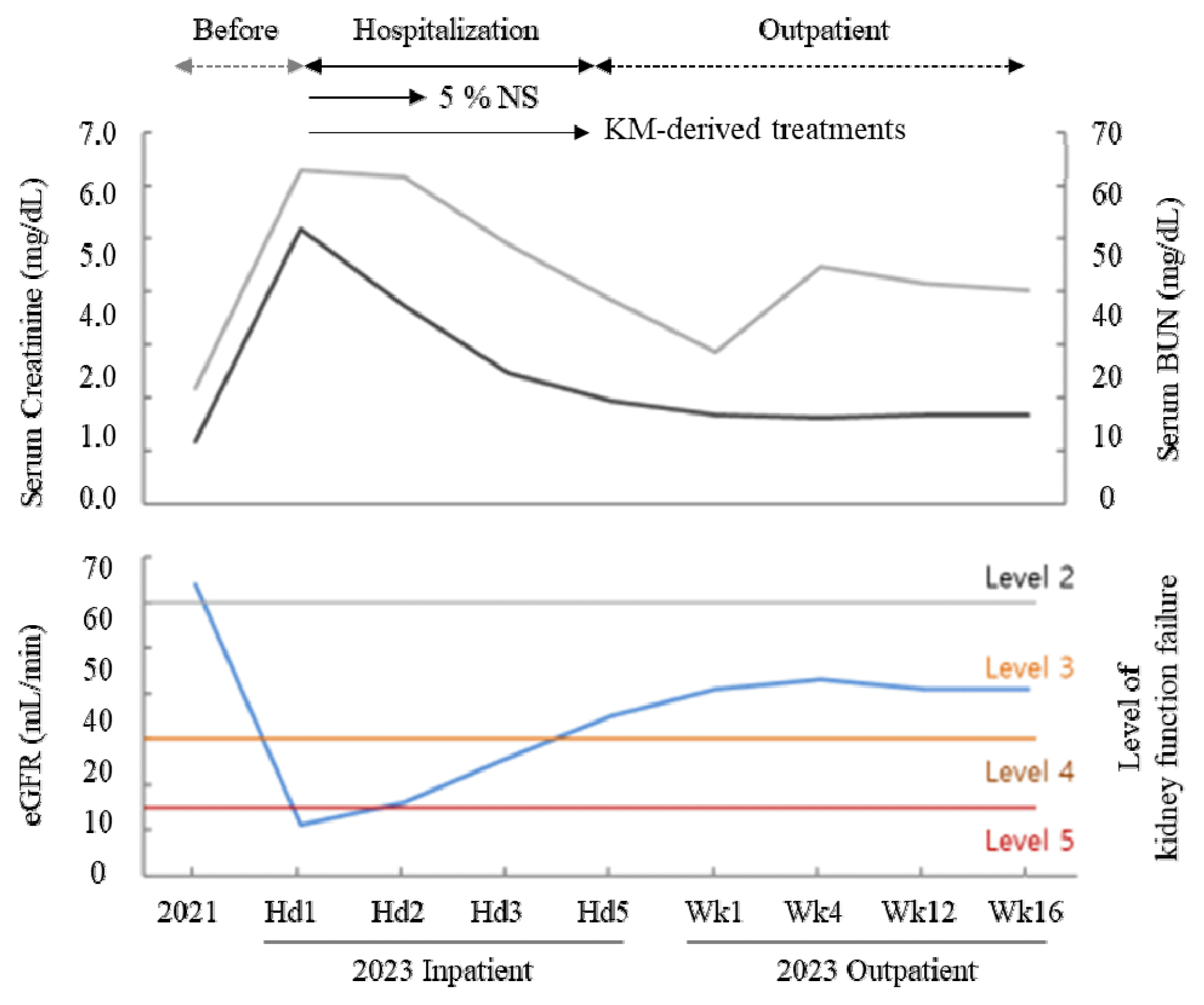
An 83-year-old male patient experienced severe diarrhea overnight, leading to hospitalization due to symptoms of dehydration and hypotension. His laboratory results displayed a typical AKI pattern, including a significant increase in creatinine levels (5.19 mg/dL) and the presence of hyperkalemia and hyponatremia. Following general treatments, including the administration of an herbal drug (Bulhwangeumjeonggi-san), the estimated glomerular filtration rate (eGFR) improved from 10 ml/min (Stage 5) to 34 ml/min (Stage 3) within five days when he was discharged. Although subsequent eGFR tests, conducted one and two months later as an outpatient, revealed an improvement of 42 ml/min, the patient still experienced mild chronic dysfunction as a consequence.
Conclusion
This study presents a noteworthy case of acute kidney injury attributed to severe dehydration, emphasizing the importance of medical awareness regarding diarrhea-induced kidney function impairment, especially in the elderly population.
Introduction
Acute kidney injury (AKI), previously referred to as acute renal failure (ARF), is characterized by a sudden and rapid decline in kidney function or kidney damage that occurs within a short timeframe1). AKI is diagnosed based on elevated serum creatinine levels (a marker of kidney excretory function) and decreased urinary output (a quantitative indicator of urine production) within 7 days, as defined by the Kidney Disease Improving Global Outcomes (KDIGO) criteria2). Given that the kidneys perform life-sustaining functions, severe AKI can be life-threatening and may necessitate kidney replacement therapy (KRT). AKI is associated with a 3- to 7-fold increase in hospital mortality, and global AKI-related mortality rates have remained high over the past five decades, surpassing those of breast cancer, heart failure, and diabetes mellitus3,4). A meta-analysis of 3,585,911 AKI cases from 154 studies reported an average pooled mortality rate of 23%5).
The causes of AKI can be categorized into three main types: prerenal AKI, which results from a severe reduction in blood flow to the kidney; intrinsic AKI, characterized by direct damage to the kidney itself; and postrenal AKI, primarily associated with urinary tract obstructions6). AKI predominantly occurs in hospitalized patients, affecting between 20.0% and 31.7% of individuals at various levels of inpatient care7). Furthermore, the epidemiological patterns of AKI vary significantly according to economic status, with hospital-acquired AKI being more common in high-income countries and community-acquired AKI in lower-income countries8).
On the other hand, the elderly is particularly susceptible to AKI due to age-related declines in renal function9). Regardless of economic status, dehydration is the most common cause of both hospital-acquired and community-acquired AKI10). For instance, one in ten adults hospitalized with diarrheal illnesses experiences AKI, with higher incidence rates among older adults11).
This study aims to present a case of AKI induced by severe diarrhea in an elderly individual, serving as a typical example of AKI, to raise awareness among practitioners in Korean medicine (KM) clinics.
Case presentation
1. Medical history and examination
An 83-year-old man, with a BMI of 28.5, had maintained good health, likely due to an active lifestyle and his occupation as a monk. He had a history of prostatomegaly and hypertension, both well-controlled with medication. However, one day, he developed diarrhea for an uncertain reason, experiencing over 20 episodes of diarrhea and mild abdominal pain over a period of 2 days. He was difficult to consume meals and felt extremely weak, prompting his visit to my clinic.
Upon his arrival at the hospital, the patient’s blood glucose levels were within the normal range (132mg/dL), but his blood pressure was alarmingly low, with a systolic reading of 85 mmHg and a diastolic reading of 56 mmHg. He had not urinated in over 20 hours and displayed symptoms of dehydration, such as intense thirst and dry mouth. During the past 2 days, he had lost approximately 2 kg in body weight. Following his hospitalization, laboratory tests revealed a significant increase in serum creatinine levels to 5.19 mg/dL and blood urea nitrogen (BUN) levels to 63.0 mg/dL. Additionally, he exhibited hyponatremia (132 mmol/L) and hyperkalemia (7.5 mmol/L), indicating the presence of acute renal failure.
2. Treatments and clinical outcome
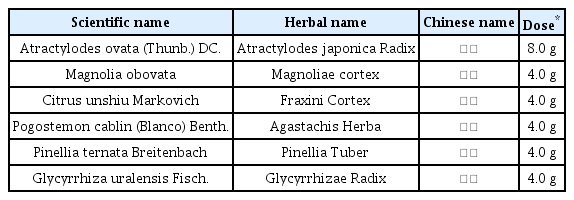
Based on the absence of any other potential causes and the previously normal results of kidney function-related tests conducted at the author’s hospital, the patient received a diagnosis of AKI attributed to volume loss and severe dehydration. His estimated glomerular filtration rate (eGFR) was calculated at 11 mL/min, placing him at the 5th level of kidney function. The primary management of this AKI involved administering 5% glucose normal saline intravascularly, along with the use of Bulhwangeumjeonggi-san syrup composed of 6 herbs (Table 1), acupuncture (both ST36, Li4, LR3), and indirect moxibustion at the Young-cheon acupoint (Ki1). Based on the rapid-weak palpitation, thirst-dry mouth and severe lethargy, the patient was diagnosed as a severe dehydration due to the continuous diarrhea. Thus Bulhwangeumjeonggi-san had been prescribed primarily to stop the diarrhea and keep the harmony of internal energy.
Laboratory tests demonstrated a rapid decrease in creatinine levels by 3.74 mg/dL after just one day of hospitalization. On the 4th day of hospitalization, his creatinine level had dropped by 1.92, indicating a 3rd level of kidney function with an eGFR of 35 mL/min. His subjective symptoms, such as faintness, malaise, and dry mouth, also improved, along with the return to normal blood pressure, sodium/potassium levels and a 1kg increase in body weight. This improvement led to his discharge from the hospital. Subsequent tests for kidney function revealed an eGFR improvement of 43 mL/min (creatinine 1.61 mg/dL) four weeks later. However, the reduced kidney function persisted for six months following discharge (Fig. 1). This case study had been approved by the Institutional Review Board for Human Research of Daejeon University Daejeon Hospital (#: DJDSKH-23-E-10-11)
Discussion and Conclusion
The present case serves as a classic example of AKI resulting from severe dehydration. Following two days of persistent diarrhea, the patient exhibited characteristic symptoms associated with volume loss, including hypotension, hyponatremia, hyperkalemia, malaise, dry mouth, a 2kg weight loss, and a day of no urination. Furthermore, the serum creatinine levels dramatically increased to 5.19 mg/dL, which was 4.4 times higher than his previous measurement of 1.18 (recorded 2 years prior). According to the KDIGO criteria for AKI, a diagnosis is based on a serum creatinine increase of ≥ 0.3 mg/dL in 48 hours, a rise of ≥ 1.5-fold from baseline within 7 days, or urine volume < 0.5 ml/kg/h for 6 hours12).
Renal tissue is highly susceptible to ischemia, and intravascular volume depletion is a common pathway in the pathophysiology of ischemic AKI13). As a result, correcting intravascular hypovolemia is a critical aspect of AKI prevention and management14). However, excessive fluid administration is associated with poor outcomes, including worsened renal function. Fluids should be administered only until intravascular hypovolemia is corrected. In this case, the patient received 2 days of intravenous 5% glucose normal saline (approximately 2L daily) and discontinued it after confirming normal blood pressure, regular urination, and correction of hyponatremia and hyperkalemia. The resolution of his diarrhea allowed for a shorter duration of intravenous fluid treatment, during which herbal medication (Bulhwangeumjeonggi-san syrup), acupuncture, and moxibustion played a significant role. The prescription of Bulhwangeumjeonggi-san and acupuncture at both ST36, Li4, and LR3 was chosen based on the its one of primary actions treating the abnormal disorders in gastrointestinal motility including diarrhea15,16). Acupoint Ki1 is the initial point of Kidney meridian and known to improve the ischemia and reperfusion of both kidneys17), while this case had been applied by an indirect moxibustion.
Although the patient’s AKI condition quickly improved from an eGFR of 11 mL/min (5th level) to 35 mL/min (3rd level) on the 4th day of hospitalization, subsequent tests indicated a decline in kidney function (Fig. 1). Despite returning to normal health with no subjective complaints, his laboratory tests revealed an increase in creatinine by approximately 1.6 mg/dL, indicating CKD corresponding to the 3rd level of kidney function (eGFR 43 mL/min). This condition persisted until 6 months after discharge (one month before now), which evidenced the development of mild chronic kidney injury. A meta-analysis reported that 25.8 out of 100 patients who had experienced AKI developed chronic kidney disease (CKD)18). In fact, the patient was already at high risk for CKD due to his age and hypertension, which had resulted in a relatively reduced eGFR of 64 mL/min (creatinine 1.16 mg/Dl) two years before the AKI episode. Alongside uncontrolled diabetes mellitus, old age and hypertension are typical risk factors for CKD18), which are corresponding to the current case.
Recent warnings have also highlighted the potential for herbal product-related nephrotoxicity19). Considering the increasing aging population with a high prevalence of diabetes mellitus and hypertension, it’s crucial to be aware of the existence of elderly individuals with impaired kidney function. According to a cohort study of 676 Italian participants aged 65 and older, a total of 33% met the criteria for CKD (GFR < 60 mL/min)20). Diarrheal diseases are common among older adults, with a longitudinal study reporting that approximately 15% of older adults (≥ 60 years) in India experienced diarrhea in the last two years21). These facts underscore the risk of kidney issues among elderly patients with diarrhea, highlighting the need for physicians to be vigilant about impaired kidney function by measuring diagnostic markers, including serum creatinine levels. Given that clinicians in the Korean medicine field may not routinely perform blood chemistry tests, awareness of the development of AKI or the progression of CKD during or after diarrhea in older adults is essential.
In summary, this report presents a typical case of diarrhea-induced AKI and its sequelae of CKD in an elderly individual. Although this case patient had been cared with herbal drug and acupuncture, it is uncertain for its direct benefit on AKI, which is a crucial limitation. Nevertheless, the present study provides a meaningful case of the severe diarrhea-induced AKI, which emphasize the importance of raising awareness about intravascular volume depletion and renal failure in especially the aging population.