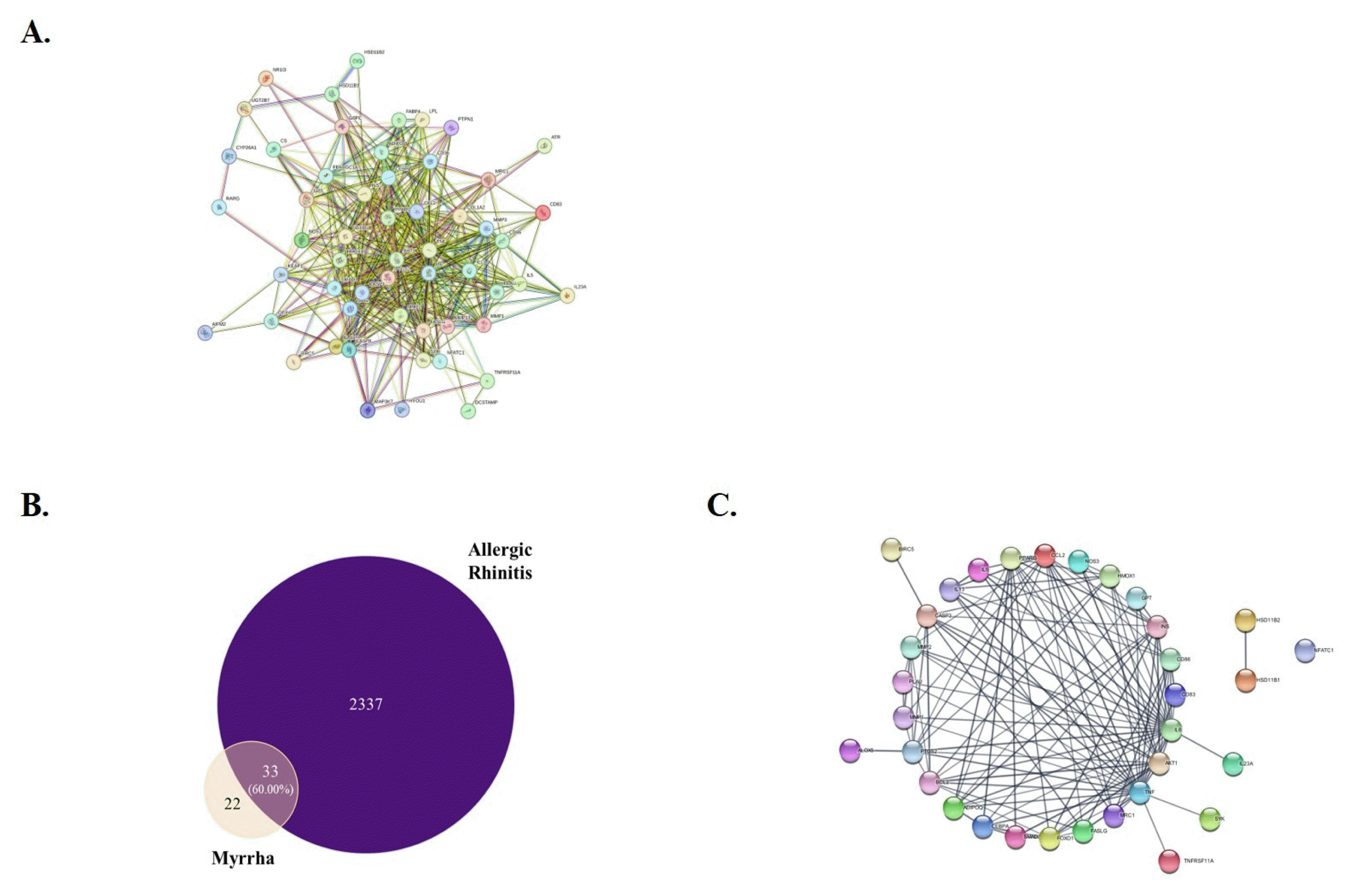
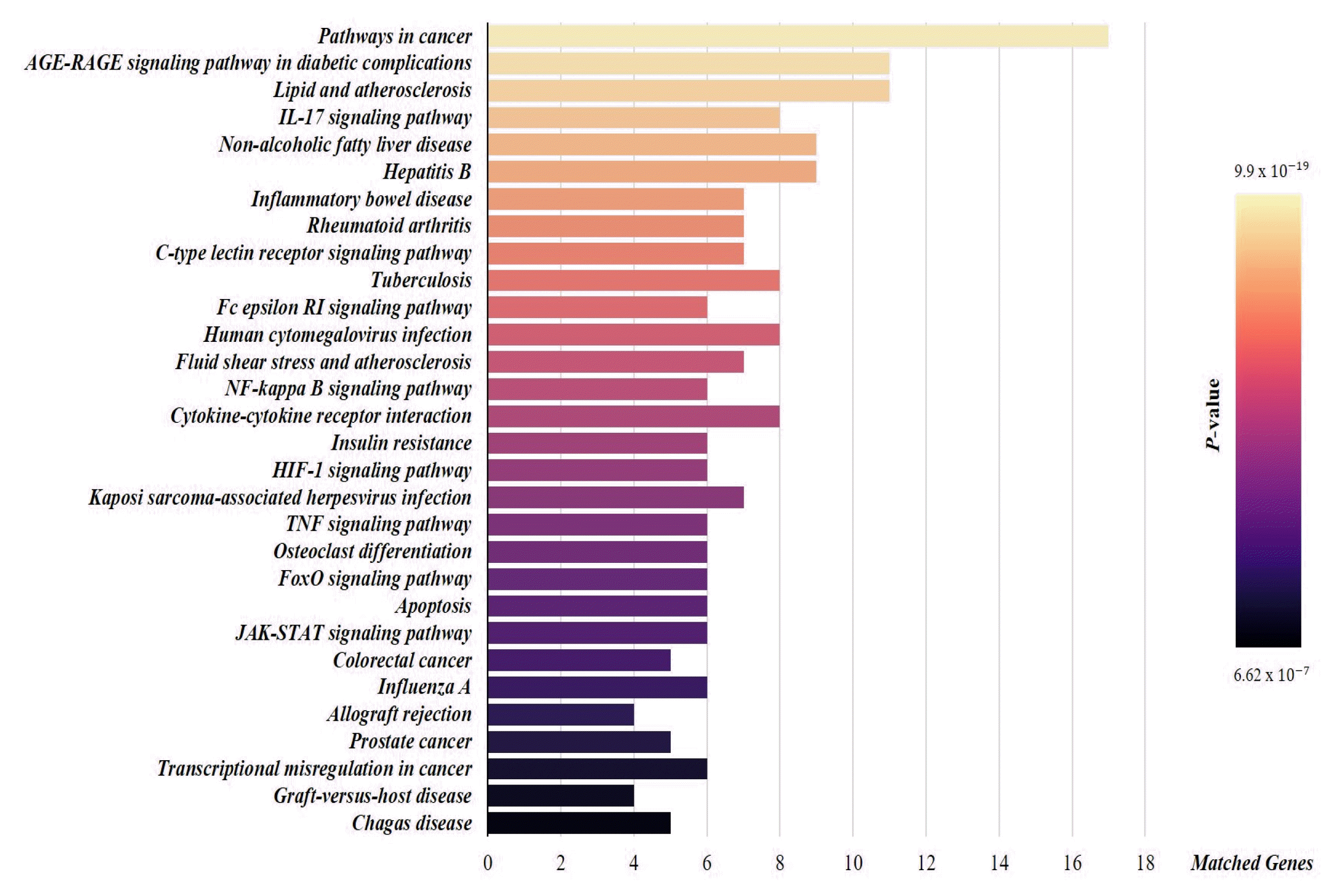
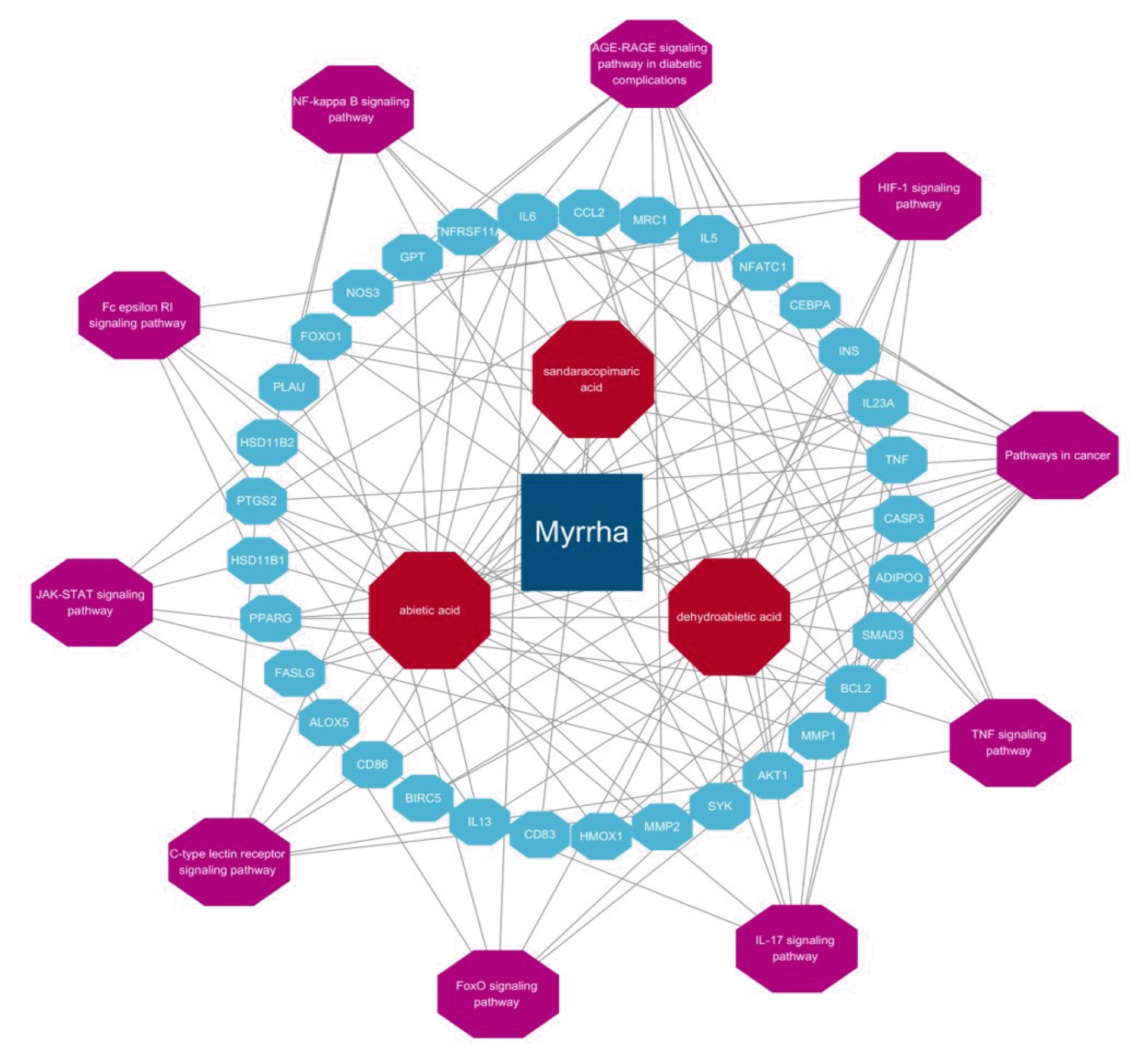
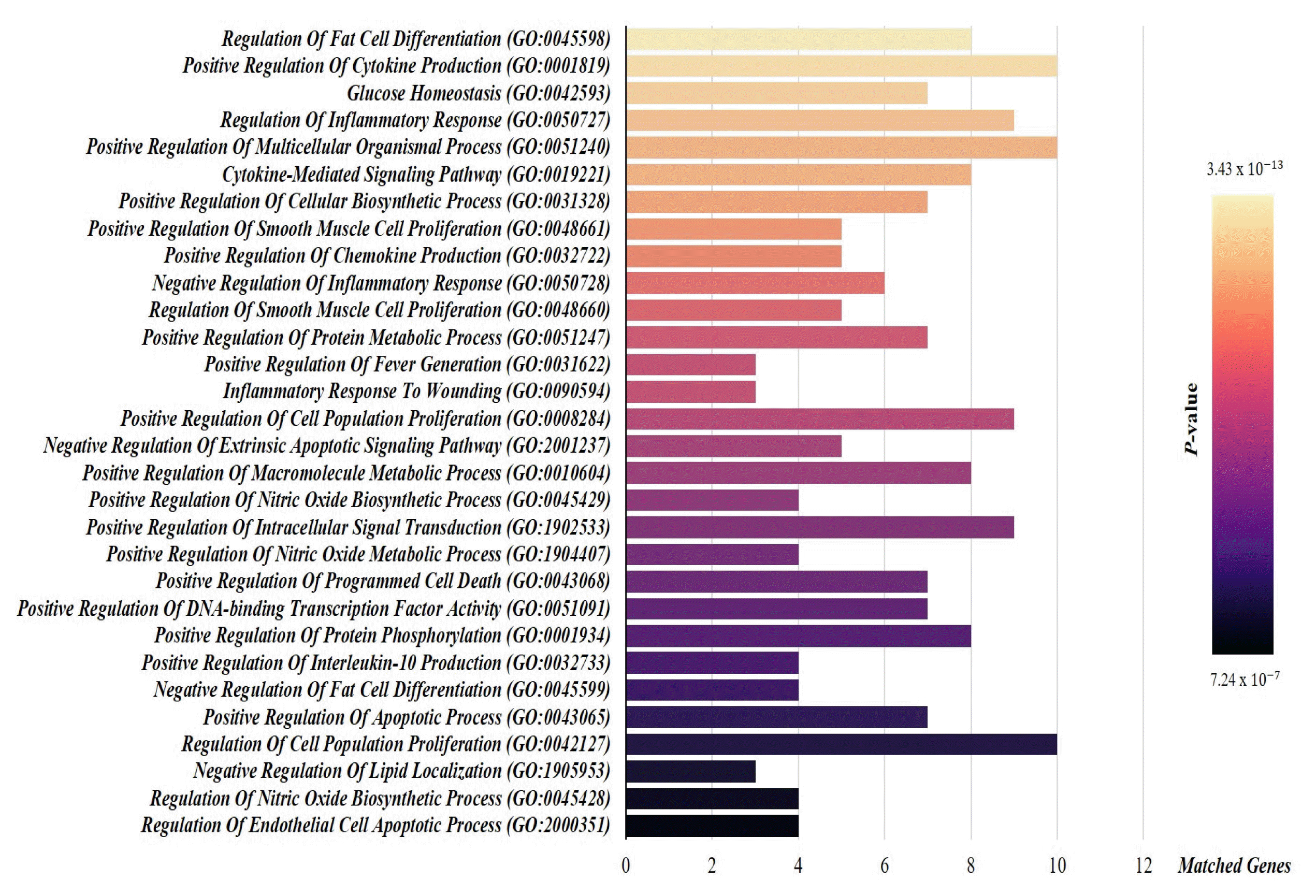References
5. Zou Q.-Y., Shen Y., Ke X., Hong S.-L., Kang H.-Y.. 2018;Exposure to air pollution and risk of prevalence of childhood allergic rhinitis: A meta-analysis. International journal of pediatric otorhinolaryngology 112(82–90)
https://doi.org/10.1016/j.ijporl.2018.06.039.
6. Kim M., Kim H.-H., Kim H.-B., Ho R. Y., Park Y., Sung M., et al. 2021;Main epidemiological characteristics and natural history of pediatric allergic rhinitis. Allergy Asthma & Respiratory Diseases 9(4):203–207.
https://doi.org/10.4168/aard.2021.9.4.203.
7. Alnahas S., Abouammoh N., Althagafi W., Abd-Ellatif E. E.. 2023;Prevalence, severity, and risk factors of allergic rhinitis among schoolchildren in saudi arabia: A national cross-sectional study, 2019. World Allergy Organization Journal 16(10):100824.
https://doi.org/10.1016/j.waojou.2023.100824.
8. herbology, T. t. c. c. o. K. 2004. Herbology Seoul: Young Lim Sa. p. 604–606.
9. Jung D.-H., Chang S.-K., Cho C.-S., Kim C.-J.. 2006;Effects of commiphora myrrha (cm) on the monosodium urate (msu)-induced gout model in rats. The Journal of Internal Korean Medicine 27(3):715–724.
10. Kim K.-S.. 2005;Acceleration of wound healing on scald burn skin using irradiation of tdp and skin spread of myrrha. J. Exp. Biomed. Sci 11(2):243–248.
11. Jung Y. H., Roh Y. W., Chong M., Jung Y. H., Roh Y. W., Chong M.. 2022;Anti-inflammatory effects of myrrh ethanol extract on particulate matter-induced skin injury. Journal of Korean Medicine 43(3):1–15.
https://doi.org/10.13048/jkm.22026.
12. Shin J. Y., Che D. N., Cho B. O., Kang H. J., Kim J., Jang S. I.. 2019;Commiphora myrrha inhibits itch-associated histamine and il-31 production in stimulated mast cells. Exp Ther Med 18(3):1914–1920.
https://doi.org/10.3892/etm.2019.7721.
15. Teng B., Zhang X., Yi C., Zhang Y., Ye S., Wang Y., et al. 2017;The association between ambient air pollution and allergic rhinitis: Further epidemiological evidence from changchun, northeastern china. International journal of environmental research and public health 14(3):226.
https://doi.org/10.3390/ijerph14030226.
16. Lee K. S., Kim K., Choi Y. J., Yang S., Kim C. R., Moon J. H., et al. 2021;Increased sensitization rates to tree pollens in allergic children and adolescents and a change in the pollen season in the metropolitan area of seoul, korea. Pediatric Allergy and Immunology 32(5):872–879.
https://doi.org/10.1111/pai.13472.
17. Lee K. S., Kim M., Kim H. H., Kim H.-B., Rha Y.-H., Park Y. M., et al. 2023;Associations between pollen and allergic rhinitis in children and adolescents. Allergy, Asthma & Respiratory Disease 11(1):3–8.
https://doi.org/10.4168/aard.2023.11.1.3.
18. Kaaaci allergic rhinitis guidelines. The korean academy of asthma, allergy and clinical immunology 2022. [cited 2024 jan 11]. Available from:
www.allergy.or.kr.
20. Kang C.-Y., Kim H.-J., Kim J.-H., Hwang J.-S., Lee D.-H.. 2021;Outcomes analysis for western medicine and korean medicine using the propensity score matching in allergic rhinitis: Data from the health insurance review and assessment service. The journal of Korean Medicine Ophthalmology & Otolaryngology & Dermatology 34(2)
https://doi.org/10.6114/jkood.2021.34.2.053.
22. Kim H. H.. 2007;Allergic rhinitis, sinusitis and asthma-evidence for respiratory system integration. Clinical and Experimental Pediatrics 50(4):335–339.
23. Chung Y. S.. 2010;Allergic rhinitis and sleep-disordered breathing. Allergy, Asthma & Immunology Research 30(4):271–274.
24. Liu X., Yang Y., Li Y., Zhang Q., Wang J., Guo J., et al. 2023;Network pharmacology-based approach for investigating the role of xanthii fructus in treatment of allergic rhinitis. Chemistry & Biodiversity 20(4):e202200785.
https://doi.org/10.1002/cbdv.202200785.
25. Shin M.. 2010. Clinical traditional herbalogy Seoul: Yeong. Lim Publishing. p. 748–749.
26. Ahmad A., Raish M., Ganaie M. A., Ahmad S. R., Mohsin K., Al-Jenoobi F. I., et al. 2015;Hepatoprotective effect of commiphora myrrha against d-galn/lps-induced hepatic injury in a rat model through attenuation of pro inflammatory cytokines and related genes. Pharmaceutical biology 53(12):1759–1767.
https://doi.org/10.3109/13880209.2015.1005754.
27. Su S., Wang T., Duan J.-A., Zhou W., Hua Y.-Q., Tang Y.-P., et al. 2011;Anti-inflammatory and analgesic activity of different extracts of commiphora myrrha. Journal of ethnopharmacology 134(2):251–258.
https://doi.org/10.1016/j.jep.2010.12.003.
29. Gibbs B. F., Räthling A., Zillikens D., Huber M., Haas H.. 2006;Initial fcɛri-mediated signal strength plays a key role in regulating basophil signaling and deactivation. Journal of allergy and clinical immunology 118(5):1060–1067.
https://doi.org/10.1016/j.jaci.2006.07.022.
31. Li Y., Leung P. S. C., Gershwin M. E., Song J.. 2022;New mechanistic advances in fcepsilonri-mast cell-mediated allergic signaling. Clin Rev Allergy Immunol 63(3):431–446.
https://doi.org/10.1007/s12016-022-08955-9.
33. Jo B. G., Bang I. S.. 2022;Anti-inflammatory effect of morinda citrifolia on lps-induced inflammation in raw 264.7 cells through the jak/stat signaling pathway. J Life Sci 32:125–134.
https://doi.org/10.5352/JLS.2022.32.2.125.
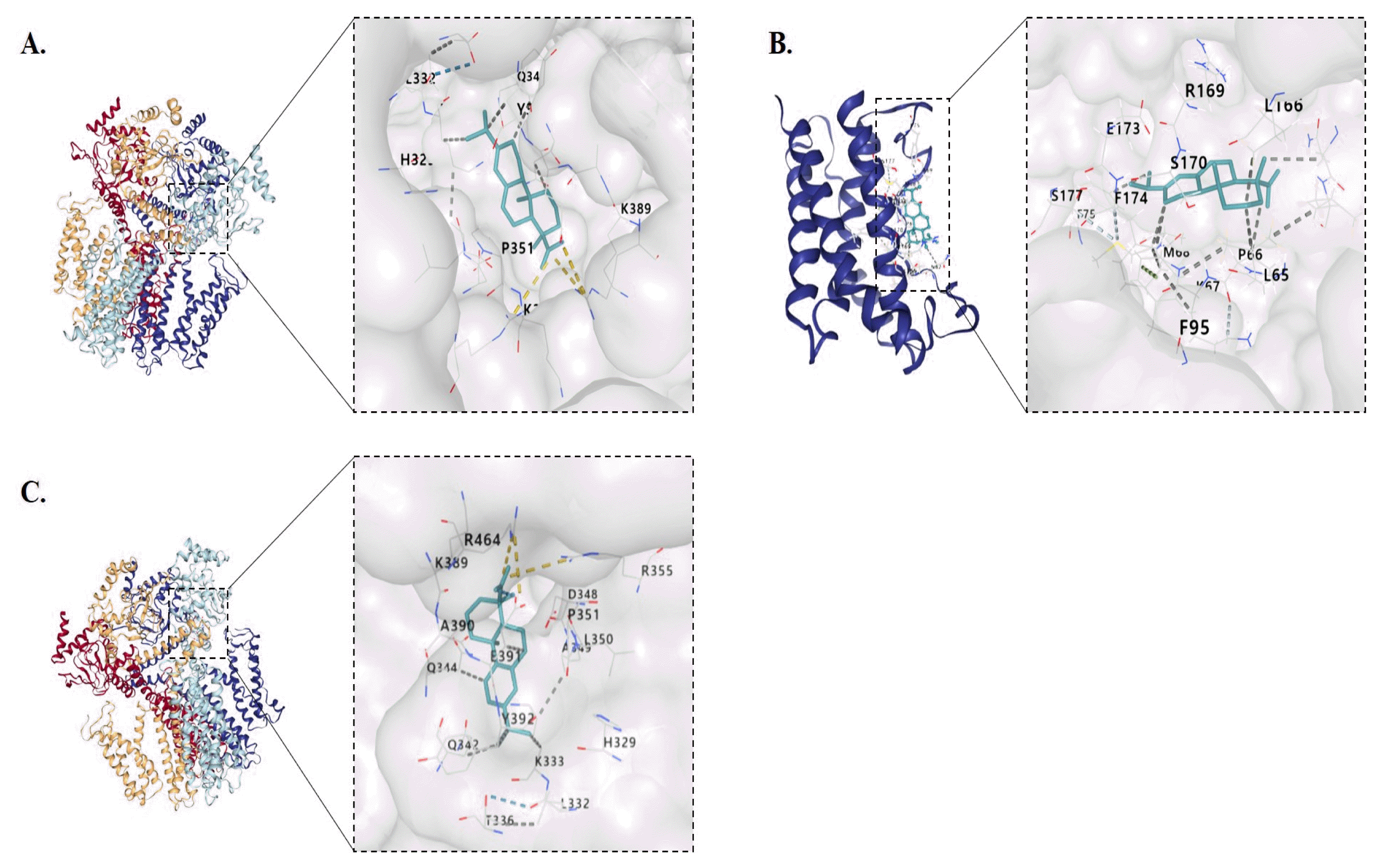
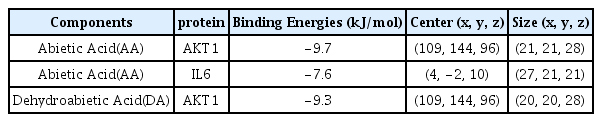
35. Di Lorenzo A., Fernández-Hernando C., Cirino G., Sessa W. C.. 2009;Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proceedings of the National Academy of Sciences 106(34):14552–14557.
https://doi.org/10.1073/pnas.0904073106.
36. Iorga B., Herlem D., Barré E., Guillou C.. 2006;Acetylcholine nicotinic receptors: Finding the putative binding site of allosteric modulators using the “blind docking” approach. Journal of molecular modeling 12(366–372)
https://doi.org/10.1007/s00894-005-0057-z.
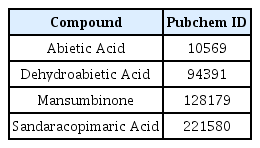
37. Gao Y., Zhaoyu L., Xiangming F., Chunyi L., Jiayu P., Lu S., et al. 2016;Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma model. Int Immunopharmacol 38:261–266.
https://doi.org/10.1016/j.intimp.2016.05.029.
38. Park J., Kim J. E., Jin Y. J., Roh Y. J., Song H. J., Seol A., et al. 2023;Anti-atopic dermatitis effects of abietic acid isolated from rosin under condition optimized by response surface methodology in dncb-spread balb/c mice. Pharmaceuticals (Basel) 16(3)
https://doi.org/10.3390/ph16030407.