Status of reports of adverse events related to botanical herbal medicines with toxic precautions officially managed by Korean government: A descriptive analysis from WHO VigiAccess
Article information
Abstract
Objectives
This study was aimed to review the global status of adverse event (AE) reports and the characteristics of the reported AEs of plants managed as herbal medicines (HMs) with toxic precautions in Korea.
Methods
This is a cross-sectional quantitative study that analyzed information available through VigiAccess, a website that provides summarized statistical information from the WHO’s global AE database to the public. VigiAccess was searched in 8 Jan, 2024. Information on the total number of reports, number of reports by year and continent, and the age and gender of patients were obtained, and the types of frequently reported AEs were also reviewed.
Results
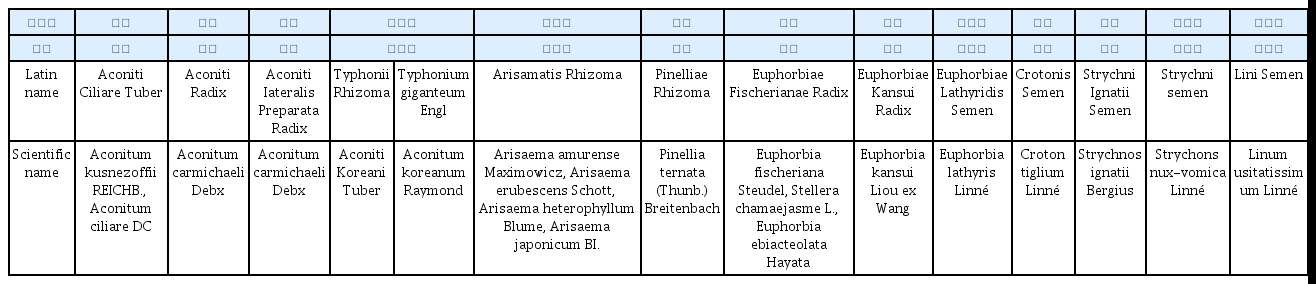
Data on the status of report submissions were obtained for a total of 9 HMs including Aconitum ciliare, Aconitum carmichaeli, Arisaema japonicum, Pinellia ternata, Euphorbiae Lathyridis, Croton tiglium, Strychni Ignatii, Strychnons nux-vomica, and Linum usitatissimum. The number of reports per HM was from 1 to 137. The most commonly reported type of AEs were gastrointestinal disorders in most of the HMs, followed by neurological disorders. Serious adverse events were reported only in Strychni Ignatii, Strychnons nux-vomica, and Linum usitatissimum, including one case of death.
Conclusions
This study shows the status of reported AEs of botanicals considered as HMs with toxic precautions in Korea based on real world data. However, when interpreting the findings of this study, readers should consider the significant limitations of this study mainly because of the characteristics of the data source.
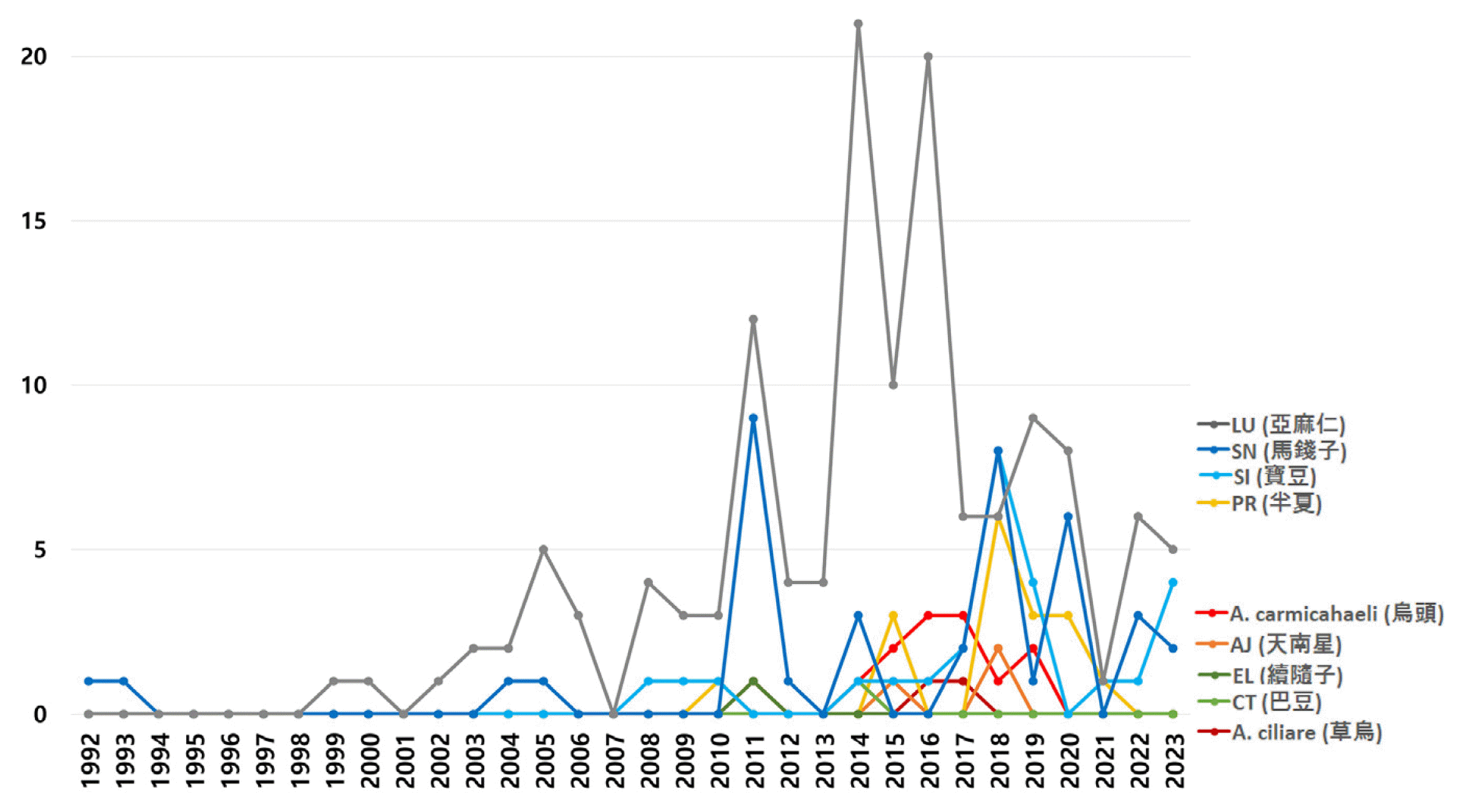
Number of annual reports for each herbal medicine
X-axis: reported year, Y-axis: number of annual reports
A. ciliare: Aconitum ciliare, A. carmichaeli: Aconitum carmichaeli, AJ: Arisaema japonicum, PT: Pinellia ternata, EL: Euphorbiae Lathyridis, CT: Croton tiglium, SI: Strychni Ignatii, SN: Strychnons nux-vomica, and LU: Linum usitatissimum
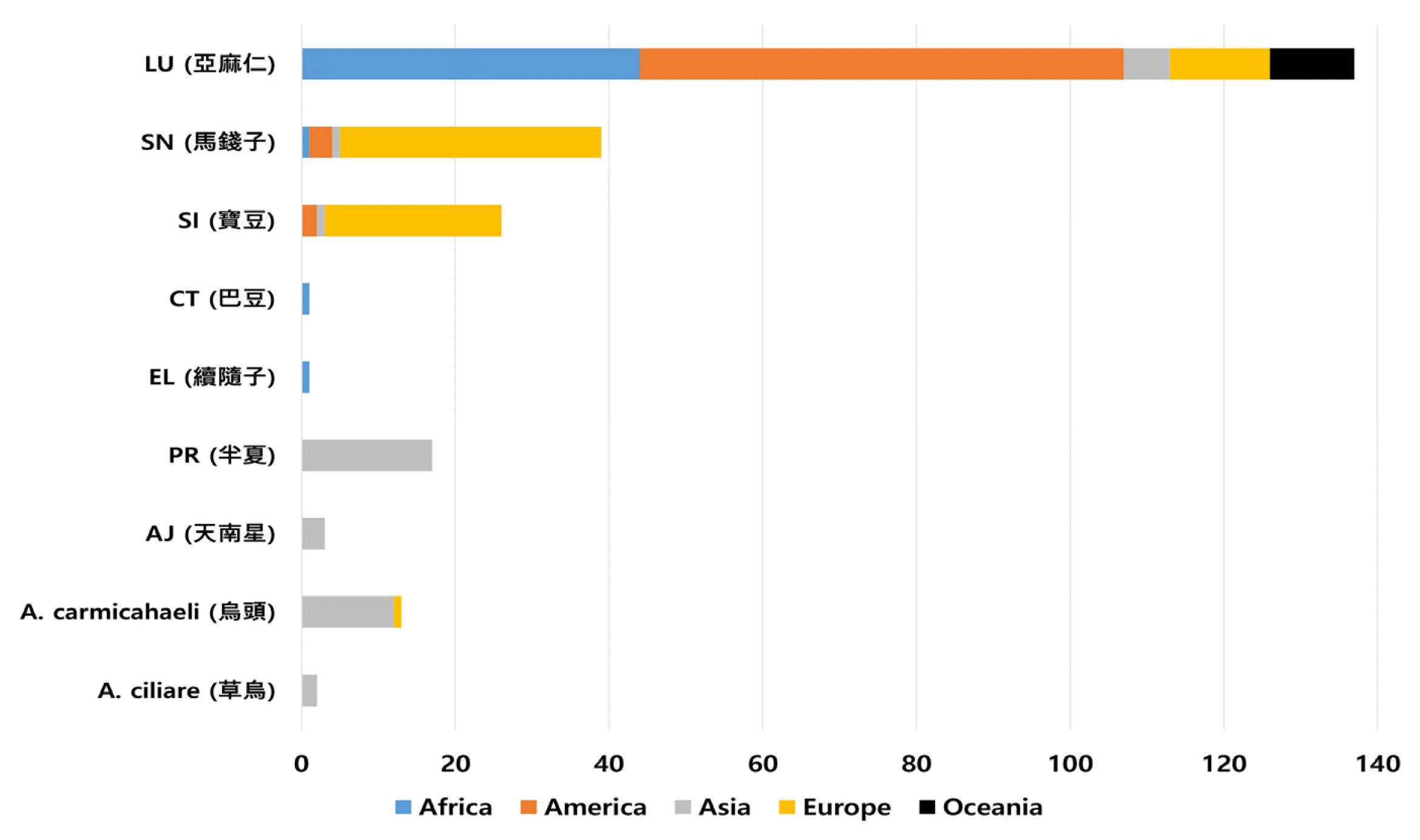
Distribution of individual case safety reports by continent
X-axis: number of cumulative reports for each herbal medicine
A. ciliare: Aconitum ciliare (n=2), A. carmichaeli: Aconitum carmichaeli (n=13), AJ: Arisaema japonicum (n=3), PT: Pinellia ternate (n=17), EL: Euphorbiae Lathyridis (n=1), CT: Croton tiglium (n=1), SI: Strychni Ignatii (n=26), SN: Strychnons nux-vomica (n=39), and LU: Linum usitatissimum (n=137)
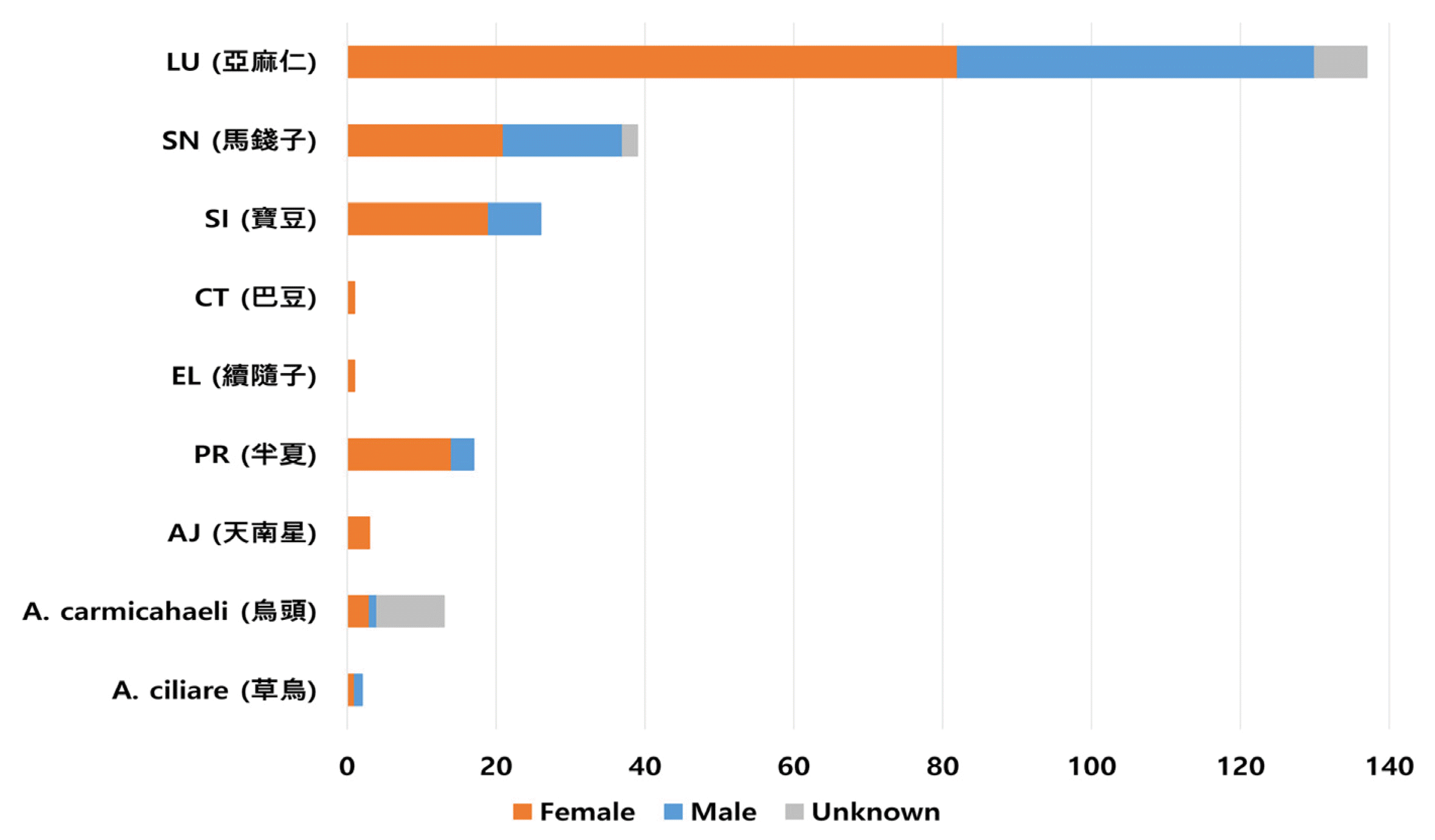
Gender distribution of patients
X-axis: total number of cumulative reports for each herbal medicine
A. ciliare: Aconitum ciliare (n=2), A. carmichaeli: Aconitum carmichaeli (n=13), AJ: Arisaema japonicum (n=3), PT: Pinellia ternate (n=17), EL: Euphorbiae Lathyridis (n=1), CT: Croton tiglium (n=1), SI: Strychni Ignatii (n=26), SN: Strychnons nux-vomica (n=39), and LU: Linum usitatissimum (n=137)
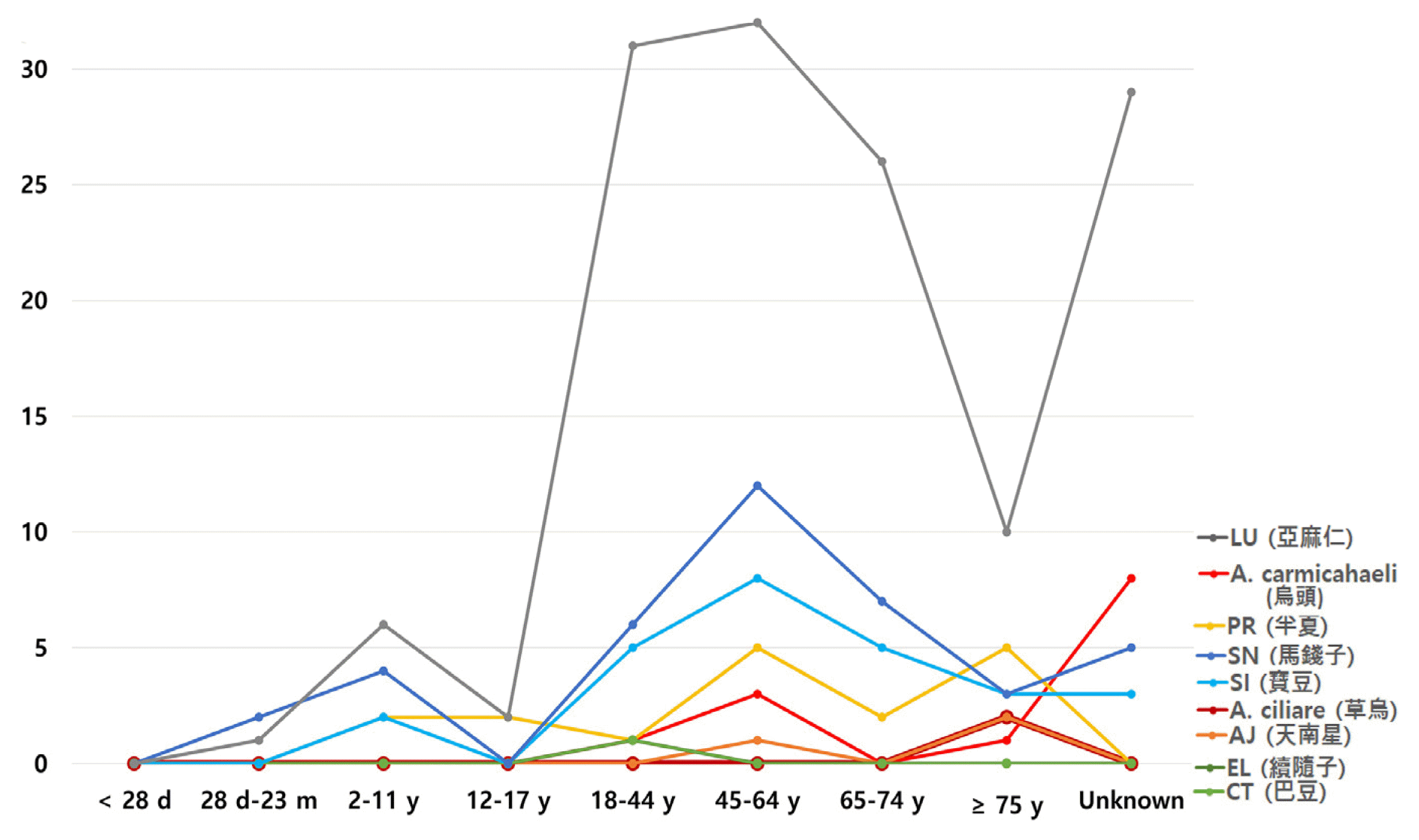
Age distribution of patients
X-axis: age range, Y-axis: number of reports for each herbal medicine
A. ciliare (n=2): Aconitum ciliare, A. carmichaeli (n=13): Aconitum carmichaeli, AJ (n=3): Arisaema japonicum, PT (n=17): Pinellia ternata, EL (n=1): Euphorbiae Lathyridis, CT (n=1): Croton tiglium, SI (n==26): Strychni Ignatii, SN (n=39): Strychnons nux-vomica, and LU (n=137): Linum usitatissimum
d: day, m: month, y: year
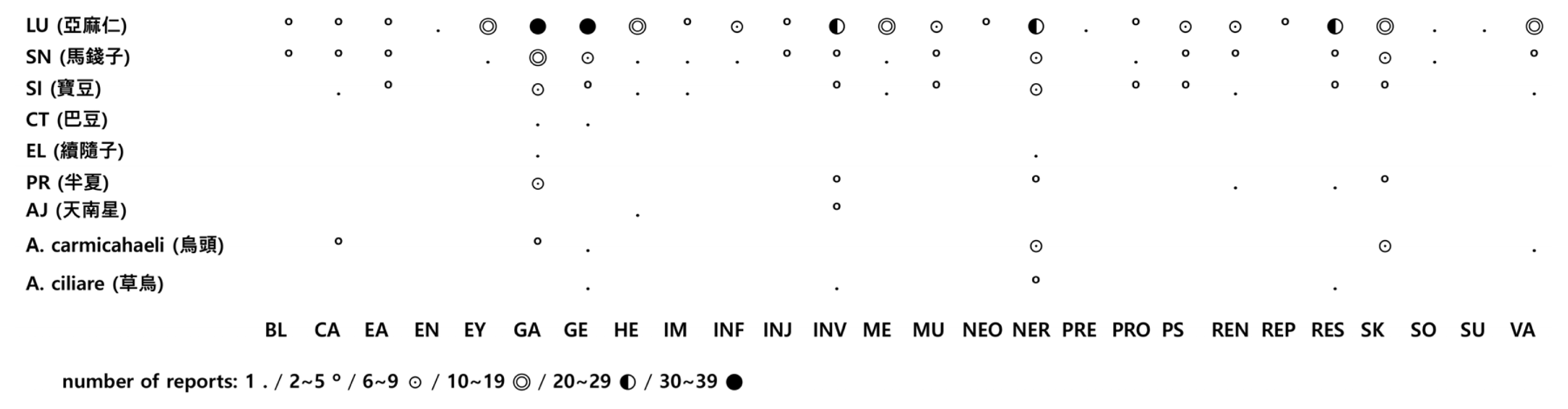
Number of Reports by System of Organ Class (SOC)
A. ciliare (n=2): Aconitum ciliare, A. carmichaeli (n=13): Aconitum carmichaeli, AJ (n=3): Arisaema japonicum, PT (n=17): Pinellia ternata, EL (n=1): Euphorbiae Lathyridis, CT (n=1): Croton tiglium, SI (n==26): Strychni Ignatii, SN (n=39): Strychnons nux-vomica, and LU (n=137): Linum usitatissimum
BL: Blood and lymphatic system disorders; CA: Cardiac disorders; EA: Ear and labrinth disorders; EN: Endocrine disorders; EY: Eye disorders; GA: Gastrointestinal disorders; GE: General disorders and administration site conditions; HE: Hepatobiliary disorders; IM: Immune system disorders; INF: Infections and infestations; INJ: Injury, poisoning and procedural complications; INV: Investigations; ME: Metabolism and nutrition disorders; MU: Musculoskeletal and connective tissue disorders; NEO: Neoplasms benign, malignant and unspecified; NER: Nervous system disorders; PRE: Pregnancy, puerperium and perinatal conditions; PRO: Product issues; PS: Psychiatric disorders; REN: Renal and urinary disorders; REP: Reproductive system and breast disorders; RES: Respiratory, thoracic and mediastinal disorders; SK: Skin and subcutaneous tissue disorders; SO: Social circumstances; SU: Surgical and medical procedures; VA: Vascular disorders