Introduction
The epidermis is the skin barrier that keeps moisture in the body and has the primary barrier function and immune response to external invading factors such as antigen and infectious agent1,2). The barrier function of the epidermis occurs through the stratum corneum structure consisting of corneocyte, cornified envelope, lamellar membrane lipid, intercorneocyte lipid, and corneodesmosome3). In the cornified envelope, the cornified protein envelope is formed by protein cross-linking such as involucrin, loricrin, trichohyalin, and small proline-rich proteins, and serves as a physical barrier4,5). The cornified lipid envelope, serves as a scaffold to induce the formation of multilamellar structures of intercellular lipid bilayer, forms a complete skin barrier structure6). In addition, filaggrin, one of the important proteins that play an important role in connecting cornified envelope and keratin intermediate microfibers, contributes to the strong physical support of the skin barrier result from acts as not only binds keratin fibers firmly but also contributes to binding proteins such as involucrin and loricrin7).
Impairment of skin barrier function correlates with inflammatory skin disease induction. Recently, reports that IL-4, IL-13 and IL-31, which are Th2 differentiation-related cytokines in atopic dermatitis, decreased the expression of proteins such as involucrin, loricrin, and filaggrin that major constituents of the skin barrier8), there is a relationship with atopic dermatitis and filaggrin gene mutation9–12), and atopic dermatitis is alleviated through improvement of skin barrier due to increased expression of filaggrin13). It could be suggested as a basis that treatment system of atopic dermatitis be diverted from based on the inhibition of Th2 skewed condition and anti-inflammation to preventive treatment system based on the improvement of skin barrier.
Hataedock is indigenous treatment of Korean medicine that administers herbal extracts orally to newborn infants for remove the fetal heat14). In Donguibogam(東醫寶鑑), when an infant is born, it is said that soft silk is immersed in a herb medicine, and then wipe away the dirt in the mouth and feed a small amount15). Among them Douchi (fermented Glycine max Merr.) is an herbal medicine that can radiate the heat of the body for reducing heat. Recently it has been reported that the isoflavone component of soybean plants alleviates inflammatory symptoms of the skin caused by heat16,17). In particular, 7, 3′, 4′-Trihydroxyisoflavone, a major metabolite of daidzein and genistein, has been reported to maintain the homeostasis of the skin barrier by increasing the expression of filaggrin in NC/Nga mice induced atopic dermatitis13).
Previous studies have shown that atopic dermatitis can be controlled after Hataedock treatment with Douchi. Excessive Th2 differentiation caused by increased IL-4 production did not occur in the NC/Nga mice treated with Hataedock. Also there were few tissue damage induced by inflammatory cytokine such as iNOS and COX-2, and few edema and itching induced by mast cell activity. It was remarkable results that the maintenance of the stratum corneum of the skin and the resulting less protein kinase C (PKC) activity18). These results could be assumed that Hataedock treatment controls the induction of atopic dermatitis by inducing the formation of the lipid barrier of the skin. However, there is no evidence on the relationship between atopic dermatitis and changes of skin barrier after Hataedock treatment.
In order to confirm the effect of the skin lipid barrier formation after the Hataedock treatment with Douchi, we observed the immunohistochemical changes of involucrin, loricrin, filaggrin, and acid sphingomyelinase (ASM). In addition, the production of cornified protein envelope such as involucrin and loricrin in the keratinocyte was confirmed by the cell line experiment. We report the significant results in promoting skin lipid barrier formation.
Method and Materials
1. Preparation and analysis of Douchi extracts
The procedure used to manufacture the herb extract for Hataedock treatment was as follows: 100 g of crushed Douchi (fermented Glycine max Merr, Namyoung Pharm., Muju, Republic of Korea) was decocted with 1000 ml of distilled water for 3 h and then filtered. The filtrate was concentrated to 50 ml under reduced pressure using a rotary evaporator, and then freeze-dried to obtain 15 g of the extract (yield: 15.0%).
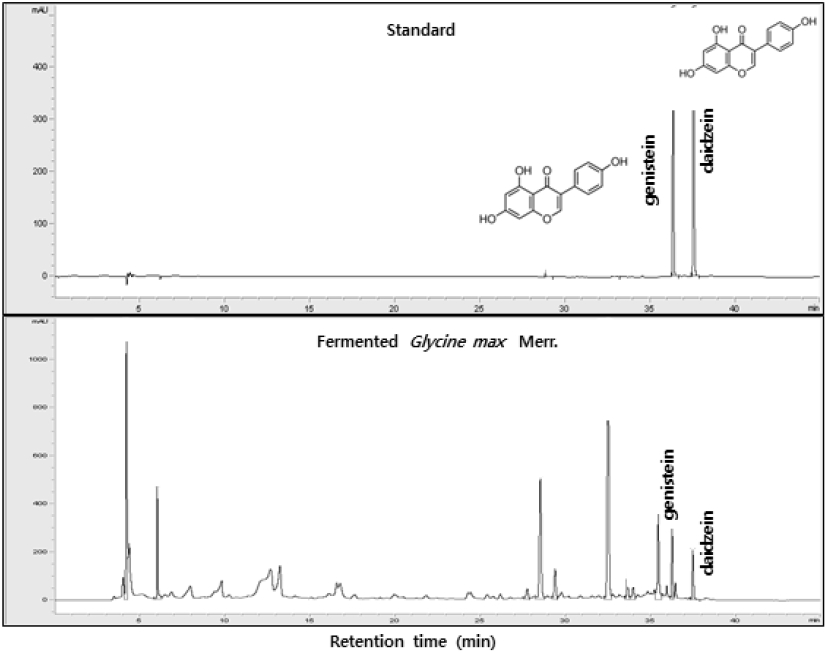
The isoflavone components of the Douchi used in this experiment were identified by HPLC and daidzein and genistein were detected (Fig. 1).
2. Western blot analysis
HaCaT keratinocytes as human keratinocyte were dispensed into the 6-well plate as 4 × 105 cells/well, cultured in 80% confluence, and then cultured for 24 h after treated with reagents. After culture, the lysate was prepared by treating with RIPA buffer (Atto, Tokyo, Japan), and centrifuged at 12,000 rpm at 4°C for 10 min to recover the supernatant. After protein quantification was performed using the Bradford protein assay reagent, 50μg of proteins were separated by SDS-polyacrylamide gel (10%) and transferred to a polyvinylidenedifluoride (PVDF) membrane at 190 mA for 80 min. The membrane was blocked in PBS containing 5% skim milk for 1 h at 37°C and treated overnight with an anti-Lorcrin antibody and anti-Involucrin antibody (# ab24722, # ab53112, Cambridge, MA, USA). In addition, it was treated with HRP-conjugated anti-rabbit antibody at room temperature for 1 h. After completion of the reaction, the membrane was developed using an enhanced chemiluminescence system (Bio Rad Laboratories, Hercules, USA) and observed for protein expression on X-ray film.
3. Hataedock treatment with Douchi
Male 3-week-old Nc/Nga mice (13–15g) were obtained from Central Lab Animal Inc. (Seoul, Republic of Korea). The mice divided into three groups (n=10 per group) as follows: the 3-week-old control group (3w-Ctrl), 5-week-old control group (5w-Ctrl), and the Hataedock-treated group (5w-FGT). Hataedock-treated group was orally administered with 10mg/kg of Douchi extracts. All procedures were conducted in accordance with IACUC approval (IACUC number: PNU-2015-0924) of Pusan National University and we followed the NIH guidelines for the care and use of laboratory animals.
4. Tissue slice and Immunohistochemistry
After 2 weeks of Hataedock treatment, sodium pentobarbital was used to anesthetize mice. Dorsal skin was fixed in 10% neutral buffered formalin (NBF) solution at room temperature for 24 h. The fixed dorsal tissue slices were embedded in paraffin, after then obtained the 5μm thick sections. The skin slices were stepped in proteinase K solution (20μg/ml) to undergo proteolysis procedure for 5 min, and then were incubated in blocking serum (10% normal mouse serum) for 2h. The slices were incubated with rabbit anti-Loricrin (1:50, Santa Cruz Biotec, USA), rabbit anti-Involucrin (1:50, Santa Cruz Biotec), rabbit anti-Filaggrin (1:100, Santa Cruz Biotec), rabbit anti-ASM (1:50, Santa Cruz Biotec), which are primary antibody, at 4°C humidified chamber for 72 h. Next the slices were linked with biotinylated mouse anti-rabbit IgG (1:100, Santa Cruz Biotec), which is a secondary antibody, for 24 h at room temperature. After the slices were exposed to the secondary antibody, an avidin biotin complex kit (Vector Lab, USA) was reacted for 1 h at room temperature. Finally, the slices were developed with 0.05 M tris-HCl buffer solution (pH 7.4), which included with 0.05% 3, 3′-diaminobenzidine and 0.01% HCl, and then contrast-stained with hematoxylin.
5. Image analysis and statistical analysis
To quantify our Immunohistochemical results, an image analysis was conducted using the Image Pro Plus (Media cybernetics, USA). The data was presented as the mean ± standard error. The skin slices that were randomly selected from each group imaged at × 400 magnification and analyzed with positive pixels/10,000,000 pixels. The data of statistics were conducted with SPSS software (SPSS 23, SPSS Inc., USA). The significance (p <0.05) was verified by using one-way Analysis of Variance (ANOVA) and post-test was performed with Duncan’s multiple range test.
Results
1. Western blot analysis
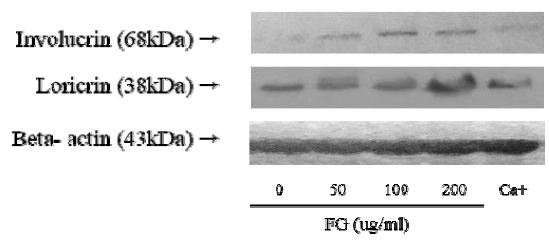
To confirm the effect of Douchi extracts on the formation of skin lipid barrier, the expression of protein such as involucrin and loricrin was measured by Western blot. Involucrin and loricrin are proteins secreted during the promotion of keratinocyte differentiation. The expression of Involucrin and loricrin was increased when keratinocyte differentiation begins, and the proteins that cross-linked by transglutaminase formed the cornified envelope protein that acts as physical barrier of skin. In particular, involucrin is forms a covalent bond with ω-hydroxyceramide in the stratum corneum, thereby forming a complete skin barrier structure with a combination of lipid membrane and the cornified envelope protein23).
It was treated with 50, 100, and 200μg/ml of Douchi extracts in western blot. As a result, the expression level of involucrin and loricrin increased at 200μg/ml concentration (Fig. 2).
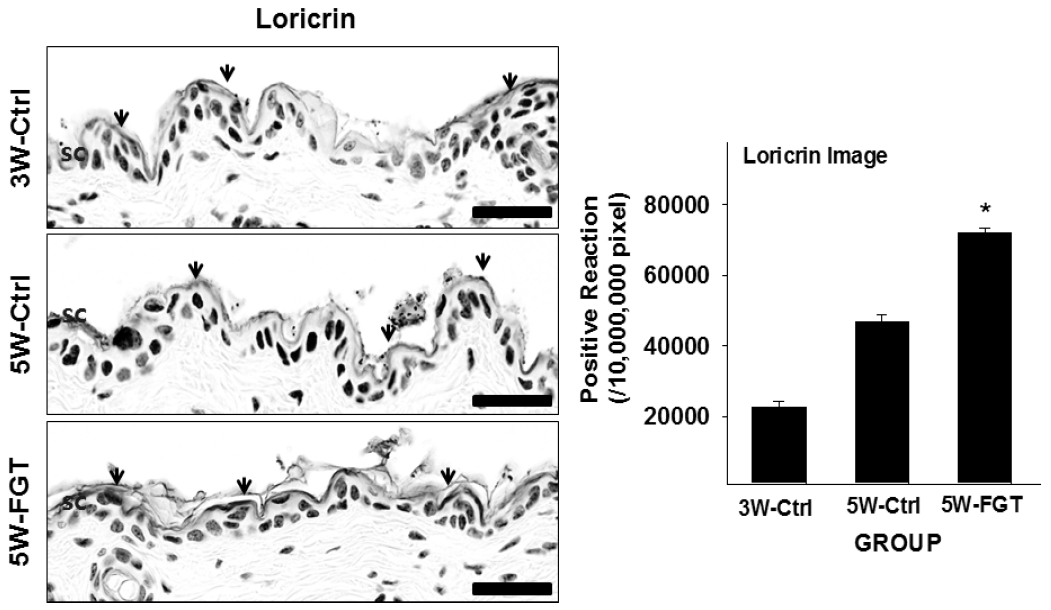
2. Positive-reaction of Loricrin
Loricrin-positive reaction in the stratum corneum was 46,742±687/10,000,000 pixel in 5W-Ctrl group, which was 108.0% higher that of 3W-Ctrl group. Whereas, in the 5W-FGT group, it was observed 71,844±481/10,000,000 pixels, which is 54% higher than the 5W-Ctrl group (Fig. 3).
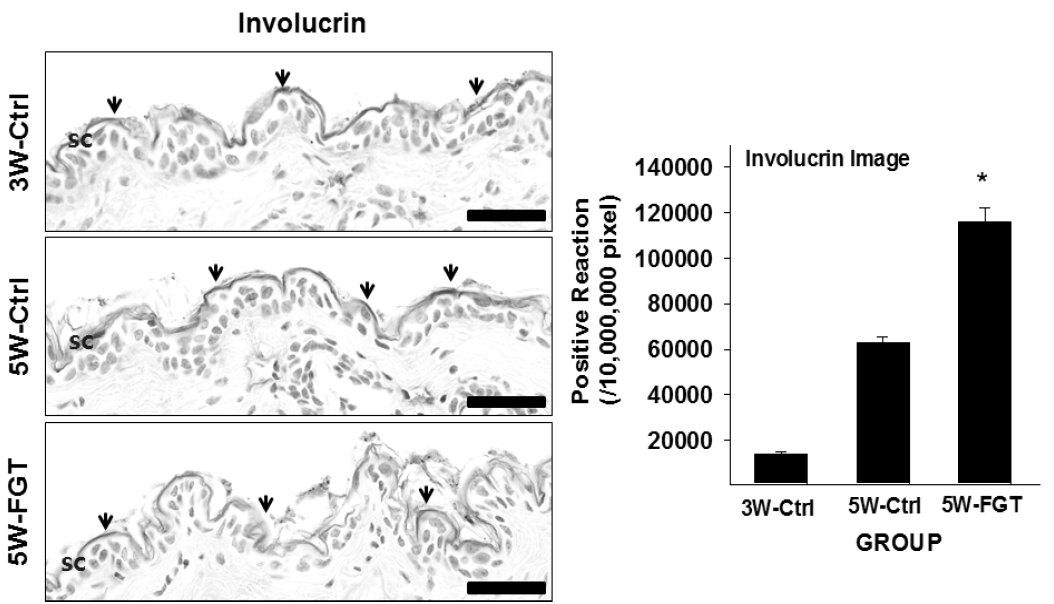
3. Positive-reaction of Involucrin
Involucrin-positive reaction in the stratum corneum was 63,117±760/10,000,000 pixel in 5W-Ctrl group, which was 1358% higher that of 3W-Ctrl group. Whereas, in the 5W-FGT group, it was observed 116,130±1,829/10,000,000 pixel, which is 84% higher than the 5W-Ctrl group (Fig. 4).
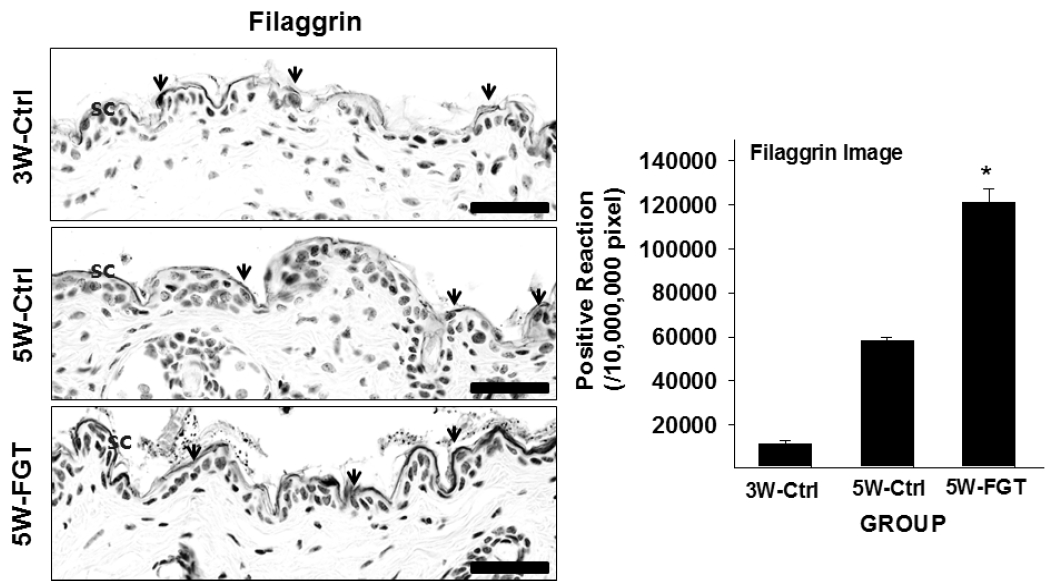
4. Positive-reaction of Filaggrin
Filaggrin-positive reaction in the stratum corneum was 58,138±522/10,000,000 pixel in 5W-Ctrl group, which was 411% higher that of 3W-Ctrl group. Whereas, in the 5W-FGT group, it was observed 120,876±2,084/10,000,000 pixel, which is 108% higher than the 5W-Ctrl group (Fig. 5).
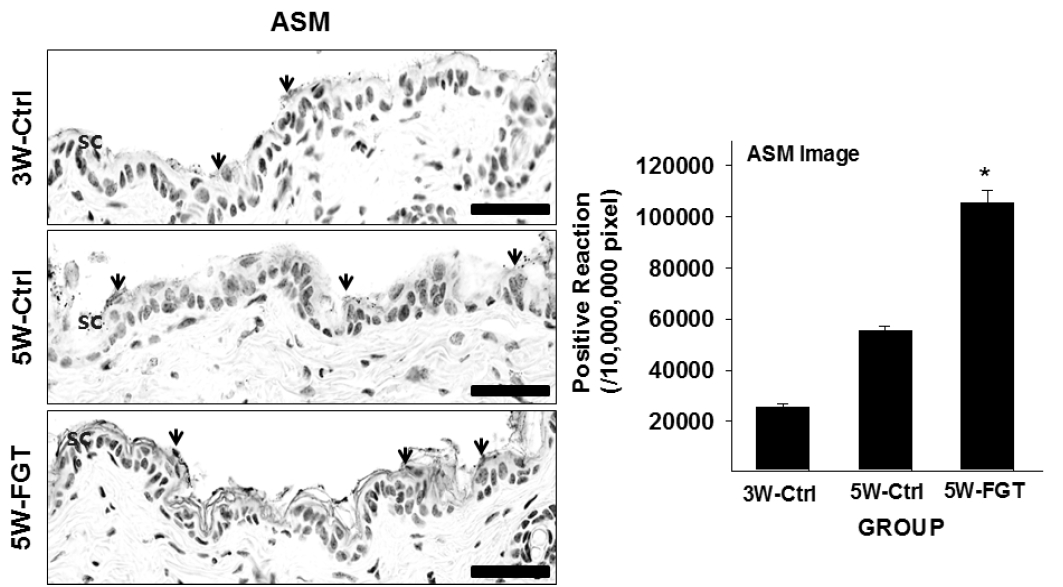
5. Positive-reaction of ASM
ASM-positive reaction in the stratum corneum was 55,246±585/10,000,000 pixel in 5W-Ctrl group, which was 117% higher that of 3W-Ctrl group. Whereas, in the 5W-FGT group, it was observed 105,261±1,567/10,000,000 pixel, which is 91% higher than the 5W-Ctrl group (Fig. 6).
6. Toxicity of the Douchi
In previous studies using the Douchi extracts, we confirmed the safety for toxicity in NC/Nga mice50), and based on these results, we administered with 10 mg/kg of Douchi orally. No significant toxicity was reported in cells or animals treated with soybean -related products and the Douchi was reported to be toxic at high doses of 2.5 g/kg/day51).
Discussion
The most important function of the skin is a role as a skin barrier that keeps moisture and prevents the penetration of pathogens and infectious substances19). Recently, studies on the relationship between skin diseases and skin barrier function have been reported20,21), and the development of therapeutic agents for skin diseases to enhance the functions of skin barrier and moisture retention has been proceeding consistently22).
The epidermis is mainly in charge of skin barrier function23). The keratinocytes in the basal layer of the epidermis are differentiated by migration to the upper layer after divisions, and these changes of epidermis are caused by the expression of the gene regulated by this process. The genes that promoting the expression are transglutaminaes 1 and 3, involucrin, loricrin, cornifin, filaggrin, and small proline-rich protein (SPR)24).
The interstitial lipids including ceramide, cholesterol and free fatty acids in stratum corneum play an important role in skin barrier and keeping moisture25).
The ceramide is a representative keeping moisture substance26), particularly, it has been reported that reduced expression in skin of atopic dermatitis27). The causes of decrease in ceramide are increased metabolism, abnormality of synthesis, and excessive decomposition28).
The free fatty acid is substances that maintain the mild acidity of pH 4.5–5.5 at epidermis and contribute to the maintenance of the homeostasis of the skin barrier29), and it has been formed by metabolizing phospholipids with secretory phospholipase A2 (sPLA2)30). Acidic pH is important role in maintaining homeostasis of skin barrier. After skin damages, it has been normal skin barrier recovery process at acidic pH. However, when pH is increased, recovery process is delayed and firmness of the stratum corneum is reduced by accelerating decomposition of corneodesmosome and activity of serine protease. It also lead to decrease function of β-glucocerebrosidase and ASM, thereby reducing the production of ceramides31).
The keratinocytes are a final products of skin differentiation process and continuously replaced with keratinocytes that have undergone a new differentiation process. In these process, the proteolytic enzyme involved in the elimination of keratinocytes are also controlled by the pH of the stratum corneum32,33). If the pH of the skin surface increases, the activity of serine protease increases. It causes an abnormal skin barrier function through promotes the elimination of keratinocytes resulting from the abnormal decomposition of the keratinocyte component31,34–35). In atopic dermatitis, the skin is not perform normal skin barrier functions because of the higher pH of the skin lesions than that of non-lesion36).
In aging skin, the most important characteristic of the cholesterol is a synthetic disorder that reported to be associated with decreased skin barrier function37).
In this study, we identify that effect of skin lipid barrier formation on Hataedock treatment with Douchi based on previous studies such as control on atopic dermatitis, maintenance of skin stratum corneum, reduction of PKC activity and reduction of epidermal cell hyperplasia.
Involucrin is one of the differentiation promoting factors of keratinocyte. It is a soluble protein with α-helix structure and the first described protein as a precursor protein that constitutes the cornified cell envelope. It is concentrated at the outermost part of the keratinocytes, is used as a marker of epidermal differentiation, and serves as a scaffold in process of cross-linking several precursor proteins38).
Loricrin expressed in the granular layer is the main protein that constitutes cornified cell envelope and accounts for 70–85% of total protein weight. The cross-linking of loricrin-loricrin, and loricrin-SPRs are play a role as strengthening cornified cell envelope 38).
Involucrin and loricrin are known to be important proteins in epidermal differentiation and skin barrier formation23). In the case of atopic dermatitis, it is confirmed that involucrin and loricrin was significantly reduced because the IL-4 and IL13 induced by the over-expressed Th2 cytokines are activating the STAT-6 signal pathway39).
Filaggrin acts as a matrix protein that aggregates keratins. It is decomposed into free amino acids by various enzymes to bind to water, thereby acting as a natural moisturizing factor. Also it has functions that regulation of pH and filter of UV40, 41). It is reported that filaggrin mutation leads to skin inflammatory reaction caused skin barrier damage in atopic dermatitis and facilitated Ig E sensitization through the damaged skin barrier. So filaggrin is typically considered to be a genetic factor of atopic dermatitis42).
ASM is an enzyme that converts sphingomyelin to ceramide. It plays a major role in the development of skin barrier. It is reported that the deficiency of ASM in atopic dermatitis causes impairment of recovery after skin barrier damage43) and the reduced activity of ASM in atopic dermatitis leads to skin barrier disorders through reducing the ceramide, involucrin, loricrin, and filaggrin44).
Traditionally, the hypothesis on the mechanism of atopic dermatitis is the inflammation of skin associated with the Type 2 immune response was predominant. Recently, it has been understood as a concept called “outside to inside”, which is the results of the skin barrier dysfunction due to the permeability of the epidermis and damage on the antibacterial barrier function45). However, an outside-inside-outside hypothesis has been suggested that inflammation of the skin caused by the immunological mechanism causes skin barrier damage again. It is reported that the skin barrier damage of atopic dermatitis induces type 2 cytokine-mediated inflammatory responses, and that induced inflammation again damages the skin barrier46). Through these studies results, renewed understanding of skin barrier abnormalities and immunological abnormalities.
Actually, it is known that Th2 cytokine reduces directly the recovery rate after skin barrier damage and suppresses the expression of filaggrin, loricrin and involucrin, which are the main components of differentiated keratinocytes. Also it suppresses the expression of antimicrobial peptide in charge of synthesizing ceramides and inherent immunity47–49).
Therefore, in this study to confirm the induction effect of the lipid barrier formation Hataedock treatment with Douchi, we measured the Western blot to observe the expression of involucrin and loricrin as components of the cornified protein envelope and also observed the positive-reaction of involucrin, loricrin, filaggrin and ASM in the stratum corneum.
As a result, it was treated with 50, 100, and 200 μg/ml of Douchi extracts in western blot, the expression level of involucrin and loricrin is increased at concentration of 200 μg/ml. In addition, the positive-reaction of involucrin, loricrin, filaggrin and ASM in the stratum corneum after Douchi treatment was significantly increased. These findings suggest that the Hataedock treatment with Douchi improved and enhanced the skin lipid barrier by the promoting differentiation of keratinocytes. Our results considered that Hataedock treatment with Douchi induces the skin barrier formation through formation and maintenance of the cornified cell envelope by increasing the expression of proteins promoted when differentiation of keratinocytes. Further studies are necessary to investigate the mechanism of keratinocytes differentiation and verify the efficacy of the Hataedock treatment.